Confocal Microscopes
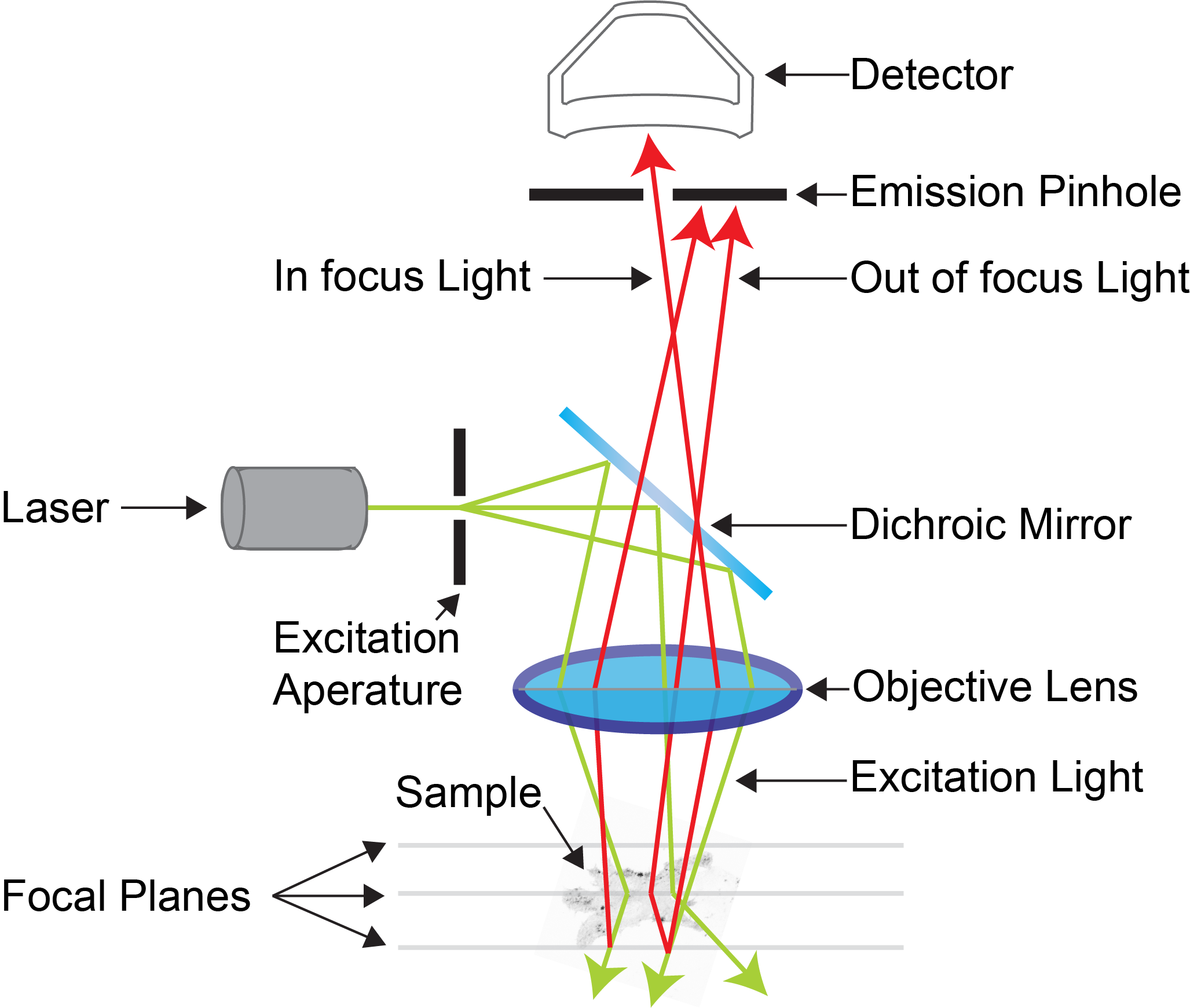
Confocal microscopy is an advanced light microscopy method which utilises a ‘pinhole’ to eliminate out of focus light and is suitable for both live and fixed cells and tissues. The advantage of a confocal microscope over conventional wide-field microscopes is that discrete optical sections can be collected while eliminating the out of focus light above and below the current plane of focus. Because of this, high powered lasers are used to illuminate the sample in order to collect enough light only from the desired focal plane.
Focused beams of laser light are scanned across the sample (Laser Scanning Microscopy, LSM) and light only from the desired focal plane is allowed to enter the detector. Depending on the fluorophores in your sample, and the number of detectors available, multiple fluorophores can be excited and detected simultaneously. Traditional detectors are photo-multiplier tubes, however more recent Gallium Arsenide Phosphide (GaAsP) detectors have been developed to increase sensitivity (Confocal 2 and 4, Airyscan; Confocal 5, BiG detectors). Leica have also developed their own higher sensitivity detector, known as a HyD - a hybrid detector combining the best properties of traditional PMTs and the highly sensitive avalanch photo-diode detectors (Confocal 1, HyD).
These microscopes provide excellent axial resolution, and very good signal to noise sampling, however this often comes with a sacrifice in temporal resolution due to the slow nature of scanning pixel-by-pixel across the image during capture. Some systems overcome this speed issue with either faster scanners (Leica SP8 - Confocal 1 uses an 8 kHz resonant scanner) or with multiple pinholes (Andor Dragonfly Spinning Disc Confocal and Yokogawa CSU-X1) solving this speed limitation in unique ways.
Confocal 1 - Inverted Leica STED 3X Confocal with FLIM
Current Status: Fully Operational
Resource Links:
- Leica STED Confocal Training Manual
- Leica Filters
- Training Videos
- Microscope location - Room 6.029a
- Make a Booking

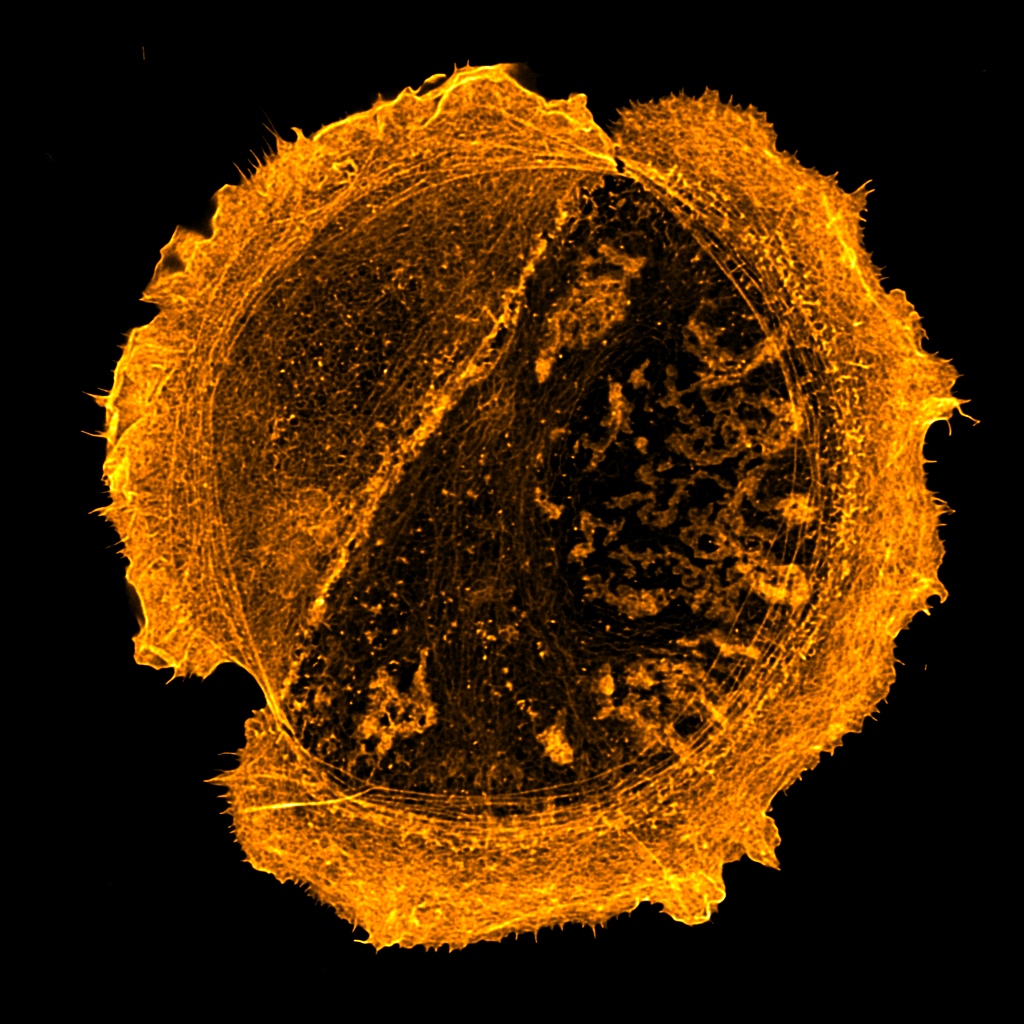
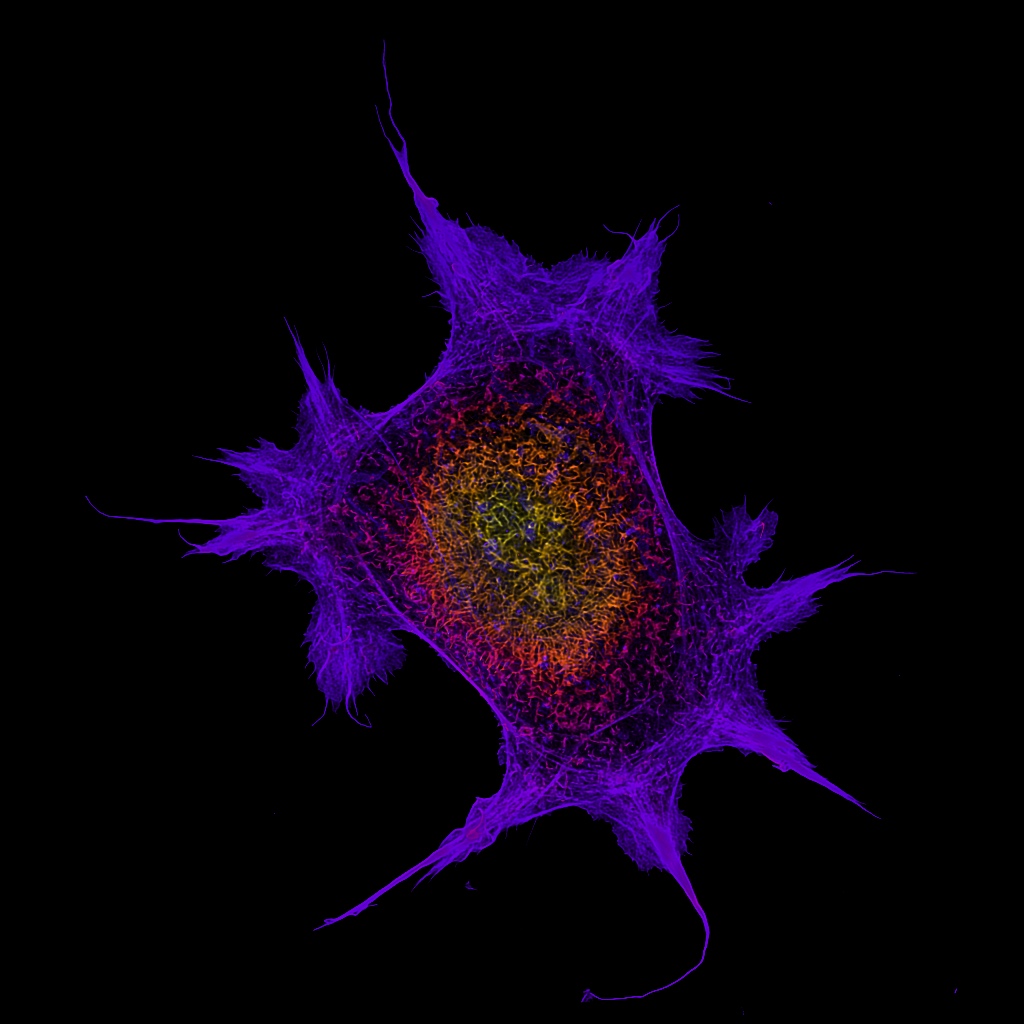
Room 6.029a - Leica DMi8 Inverted Microscope Stand with SP8 Galvo and Resonant Confocal Scanners, White Light Laser (WLL), STED 3X super-resolution, and FLIM/FRET
Inverted point-scanning laser confocal microscope with spectral, super resolution and FLIM/FRET detection. Tuneable 470nm - 670nm WLL, 2 x PMT and 2 x HyD detectors. Suited for imaging fixed samples on slides, samples with low fluorescence and live imaging.
Features Include:
- Fully motorised X-Y-Z stage
- High-sensitivity HyD detectors
- CO2 and temperature incubation for live cells and tissue
- Tiled imaging
- Multi-position imaging
- Spectral imaging
- Super-Resolution Imaging with 592nm, 660nm and 775nm STED lasers
- Fast Resonant Imaging
- FLIM/FRET Imaging with WLL and 440nm pulsed lasers
Transmitted Light: Leica LED Lamp
Reflected Light: Leica EL6000 120W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| Universal LWD | 0.55 | 28 | - | K3 | K6 | K7 | - | - |
Objectives:
| Position | Objective | Magnification | N.A. | Immersion | W.D. (mm) | Type | Type | Thread |
| 1 | HC Plan Apochromat | 10x | 0.4 | Dry | 2.2 | DIC | ||
| 2 | HC Plan Apochromat | 40x | 0.85 | Air | 0.21 | DIC | ||
| 3 | HC Plan Apochromat | 40x | 1.1 | Water | 0.65 | DIC | ||
| 5 |
HC Plan Apochromat(*) |
93x | 1.30 | Glyc | 0.3 | DIC | ||
| 6 | HC Plan Apochromat | 100x | 1.40 | Oil | 0.13 | DIC |
* Note: The 93x objective also comes with a motorised correction collar. A 20x Air and 20x Korr are both available upon request.
Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
| 1 |
DAPI |
350/50 |
|
460/50 |
DAPI, Hoechst |
| 2 |
GFP |
470/40 |
|
525/50 |
EGFP, A488 |
| 3 |
YFP |
500/20 |
|
535/30 |
YFP |
| 4 | Texas Red | 560/40 | 630/75 | Texas Red, mCherry | |
Lasers:
|
Laser |
Wavelength |
Laser Power |
BFP Power* |
Suitable Dyes |
|
405 |
405nm |
50mW |
- |
DAPI / Hoechst |
|
440 pulsed |
440nm |
- |
- |
CFP FLIM/FRET |
|
442 |
442nm | 40mW | - | CFP |
| WLL |
470nm-670nm |
1.5mW |
- |
Visible Dyes |
|
92 STED |
592nm |
1.5W |
- |
Star 488, Oregeon Green |
|
660 STED |
660nm |
1.5W |
- |
Star 470sx, A546 |
|
775 STED |
775nm |
1.5W |
- |
SiR |
*BFP = Back Focal Plane of Objective
Computer:
HP Z640 PC - 3GHz Xeon Processor - 64GB RAM - 24GB nVidia Quadro M6000 GPU
3TB Raid Array
2 x 30" LCD Monitor
Software:
Leica LASX Software
Co-localisation, 3D visualisation, LAS Navigator, FRAP, FRET, FALCON FLIM and LIGHTNING Deconvolution.
Accessories:
Incubation chamber with CO2 and Temperature control
Confocal 2 - Inverted LSM 880 Fast Airyscan
Resource Links:

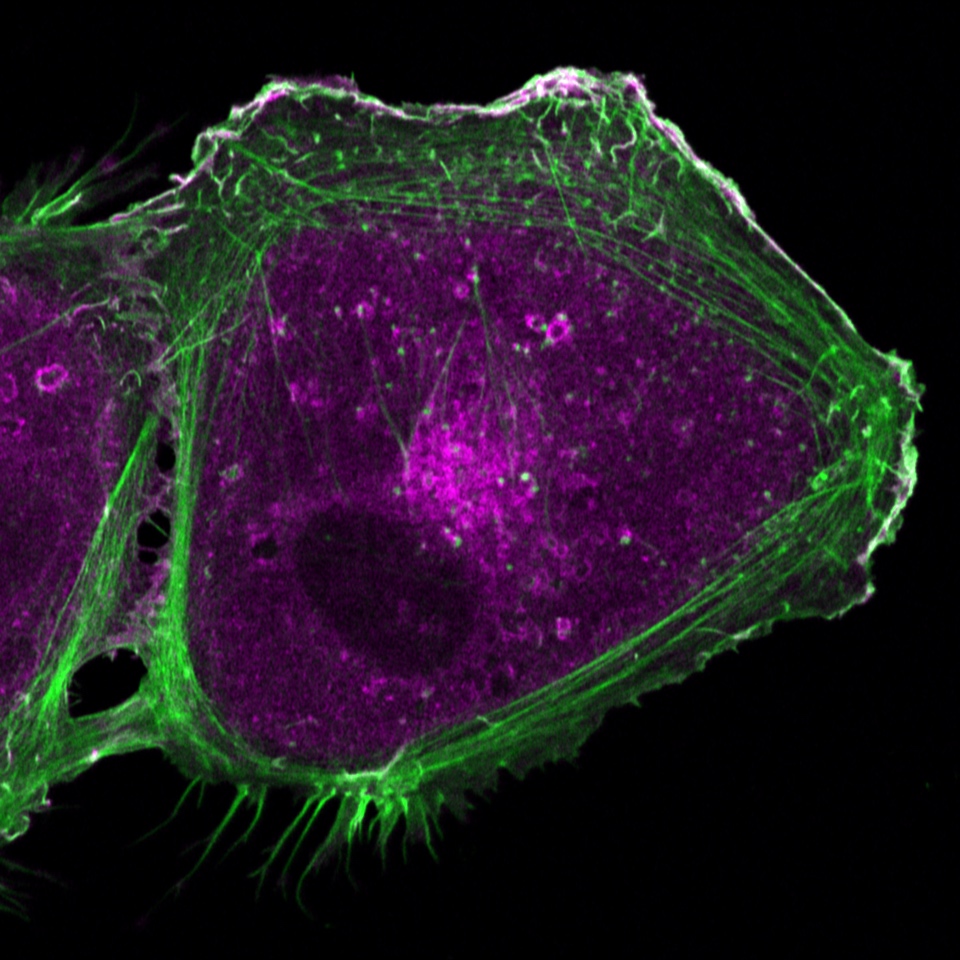
Room 6.029 - Zeiss Axiovert 200 Inverted Microscope Stand with LSM 880 Confocal Scanner with Fast Airyscan Detector
Inverted point-scanning laser confocal microscope with spectral detection. Suited for imaging fixed samples on slides, samples with low fluorescence, super-resolution Airyscan detection and high-speed live imaging.
Features Include:
- Fully motorised X-Y-Z stage
- Tiled imaging
- Multi-position imaging
- Spectral imaging
- Airyscan super-resolution
- Fast airyscan high-speed imaging
Transmitted Light: Zeiss 100W Halogen Lamp
Reflected Light: HBO 100W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| - | - | - | - | - | - | - | - | - |
Objectives:
| Position | Objective | Magnification | N.A. | Immersion | W.D. (mm) | Type | Type | Thread |
| 1 | Plan Apochromat | 10x | 0.45 | Dry | - | - | M27 | |
| 2 | Plan Apochromat | 20x | 0.80 | Dry | - | - | - | M27 |
| 3 | Plan Apochromat | 40x | 1.3 | Oil | - | - | M27 | |
| 4 | Plan Apochromat | 63x | 1.4 | Oil | - | - | M27 |
Note: the 63x Oil objective is hand-picked to be used with the Airy-scan detector as well as a separate 40x Water objective that is also available.
Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
| 1 | Zeiss #63 HE | 572/25 | FT 590 | 629/62 | mCherry, Texas Red |
| 2 | Zeiss #38 HE | 470/40 | FT 495 | 525/50 | EGFP, Alexa Fluor 488 |
| 3 | Zeiss #47 HE | 436/25 | FT 455 | 480/40 | DAPI, Hoechst |
| 4 | - | - | - | - | - |
| 5 | - | - | - | - | - |
Lasers:
|
Laser |
Wavelength |
Laser Power |
BFP Power* |
Suitable Dyes |
|
405 |
405nm |
- |
- |
DAPI / Hoechst |
|
Argon Ion |
458nm |
- | - |
CFP |
|
Argon Ion |
488nm |
- | - |
GFP / Alexa Fluor488 |
|
Argon Ion |
514nm |
- | - |
YFP |
|
561 |
561nm |
- | - |
mCherry / Cy3 |
|
633 |
633nm |
- | - |
Cy5 / A647 |
*BFP = Back Focal Plane of Objective
Computer:
Fujitsu PC - 3GHz Xeon Processor - 32GB RAM - 1GB nVidia Quadro GPU
3TB Raid Array
30" LCD Monitor
Software:
Zeiss Zen 2012 Black
Accessories:
Incubation chamber with CO2 and Temperature control
Confocal 3 - Upright LSM 710 Meta
Resource Links:

Room 6.019a - Zeiss Axiovert 200 Upright Microscope Stand with LSM 710 Meta Confocal Scanner
Upright point-scanning laser confocal microscope with spectral detection Suited for imaging fixed samples on slides, samples with low fluorescence and live imaging.
Features Include:
- Fully motorised X-Y-Z stage
- Tiled imaging
- Multi-position imaging
- Spectral imaging
Transmitted Light: Zeiss 100W Halogen Lamp
Reflected Light: Exfo Xcite 120W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| - | - | - | - | - | - | - | - | - |
Objectives:
| Position | Objective | Magnification | N.A. | Immersion | W.D. (mm) | Type | Type | Thread |
| 1 | Plan Apochromat | 10x | 0.45 | Dry | - | - | M27 | |
| 2 | Plan Apochromat | 20x | 0.80 | Dry | - | - | - | M27 |
| 3 | Plan Apochromat | 40x | 1.3 | Oil | - | - | M27 | |
| 4 | Plan Apochromat | 63x | 1.4 | Oil | - | - | M27 |
Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
| 1 | Zeiss #63 HE | 572/25 | FT 590 | 629/62 | mCherry, Texas Red |
| 2 | Zeiss #38 HE | 470/40 | FT 495 | 525/50 | EGFP, Alexa Fluor 488 |
| 3 | Zeiss #47 HE | 436/25 | FT 455 | 480/40 | DAPI, Hoechst |
| 4 | - | - | - | - | - |
| 5 | - | - | - | - | - |
Lasers:
|
Laser |
Wavelength |
Laser Power |
BFP Power* |
Suitable Dyes |
|
405 |
405nm |
- |
- |
DAPI / Hoechst |
|
Argon Ion |
458nm |
- | - |
CFP |
|
Argon Ion |
488nm |
- | - |
GFP / Alexa Fluor488 |
|
Argon Ion |
514nm |
- | - |
YFP |
|
561 |
561nm |
- | - |
mCherry / Cy3 |
|
633 |
633nm |
- | - |
Cy5 / A647 |
*BFP = Back Focal Plane of Objective
Computer:
Fujitsu PC - 3GHz Xeon Processor - 32GB RAM - 1GB nVidia Quadro GPU
3TB Raid Array
30" LCD Monitor
Software:
Zeiss Zen 2012 Black
Accessories:
Incubation chamber with CO2 and Temperature control
Confocal 4 - Inverted LSM 710 Meta NLO AiryScan
Resource Links:
- Confocal 4 Training Manual
- Mai Tai Multiphoton Laser Training Manual
- AiryScan Training Manual
- Zeiss Filters
- Microscope location - Room 6.034
- Make a Booking

Room 6.034 - Zeiss Axiovert 200 Inverted Microscope Stand with LSM 710 Meta Confocal Scanner
Inverted point-scanning laser confocal microscope with spectral detection and Airyscan super resolution detector. 2-photon imaging with a fully tunable Mai Tai eHP DeepSee 760-1040nm laser with dedicated BiG(GaAsP) NDD detectors for high sensitivity. Suited for imaging fixed samples on slides, samples with low fluorescence and live imaging.
Features Include:
- Fully motorised X-Y-Z stage
- Piezo Z-drive for rapid Z-stacks
- High-sensitivity BiG detectors (GFP/mCherry as wel as a dedicated SHG filter) for 2-photon imaging
- AiryScan detector for super resolution imaging to 130nm (XY)
- CO2 and temperature incubation for live cells and tissue
- Tiled imaging
- Multi-position imaging
- Spectral imaging
Transmitted Light: Zeiss 100W Halogen Lamp
Reflected Light: Exfo Xcite 120W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| Universal LWD | 0.55 | 26 | - | - | - | - | - | - |
Objectives:
| Position | Objective | Magnification | N.A. | Immersion | W.D. (mm) | Type | Type | Thread |
| 1 | Plan Apochromat | 20x | 0.8 | Dry | - | - | M27 | |
| 2 | Plan Apochromat | 40x | 1.3 | Oil | - | - | M27 | |
| 3 | Plan Apochromat | 63x | 1.40 | Oil | - | - | M27 | |
| 4 | Plan Apochromat | 100x | 1.46 | Oil | - | - | M27 | |
| 5 | - | - | - | - | - | - | M27 | |
| 6 | Plan Apochromat | 10x | 0.45 | Dry | - | - | M27 |
Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
| 1 |
Zeiss #63 HE |
572/25 |
FT 590 |
629/62 |
mCherry, Texas Red |
| 2 |
Zeiss #38 HE |
470/40 |
FT 495 |
525/50 |
EGFP, Alexa Fluor 488 |
| 3 |
Zeiss #47 HE |
436/25 |
FT 455 |
480/40 |
DAPI, Hoechst |
| 4 | - | - | - | - | - |
| 5 | - | - | - | - | - |
Lasers:
|
Laser |
Wavelength |
Laser Power |
BFP Power* |
Suitable Dyes |
|
405 |
405nm |
20mW |
4.5mW |
DAPI / Hoechst |
|
Argon Ion |
458nm |
1mW |
0.4mW |
CFP |
|
Argon Ion |
488nm |
20mW |
8mW |
GFP / A488 |
|
Argon Ion |
514nm |
3mW |
1mW |
YFP |
|
561 |
561nm |
10mW |
5mW |
mCherry / Cy3 |
|
633 |
633nm |
5mW |
1mW |
Cy5 / A647 |
|
Mai Tai |
780 - 1040 nm |
2000mW |
10mW - 50mW |
2 Photon Dyes |
*BFP = Back Focal Plane of Objective
Computer:
Fujitsu PC - 3GHz Xeon Processor - 32GB RAM - 1GB nVidia Quadro GPU
3TB Raid Array
30" LCD Monitor
Software:
Zeiss Zen 2012 Black
Accessories:
Incubation chamber with CO2 and Temperature control
Confocal 5 - Inverted LSM 710 BIG
Resource Links:
- Confocal 5 Training Manual
- Zeiss Filters
- Training Videos
- Microscope location - Room 6.028
- Make a Booking

Room 6.028 - Zeiss Axiovert 200 Inverted Microscope Stand with LSM 710 Confocal Scanner
Inverted point-scanning laser confocal microscope. Dedicated BiG(GaAsP) detectors for increased sensitivity. Suited for imaging fixed samples on slides, samples with low fluorescence and live imaging.
Features Include:
- Fully motorised X-Y-Z stage
- High-sensitivity BiG detectors (GFP/mCherry and CFP/YFP)
- CO2 and temperature incubation for live cells and tissue
- Tiled imaging
- Multi-position imaging
- Spectral imaging
Transmitted Light: Zeiss 100W Halogen Lamp
Reflected Light: Exfo Xcite 120W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| Universal LWD | 0.55 | 26 | - | - | - | - | - | - |
Objectives:
| Position | Objective | Magnification | N.A. | Immersion | W.D. (mm) | Type | Type | Thread |
| 1 | Plan Apochromat | 10x | 0.45 | Dry | - | - | M27 | |
| 2 | Plan Apochromat | 20x | 0.8 | Dry | - | - | M27 | |
| 3 | EC Plan-Neofluar | 40x | 0.75 | Dry | - | - | M27 | |
| 4 | LD C-Apochromat | 40x | 1.1 | Water | - | - | M27 | |
| 5 |
LD C-Apochromat |
63x | 1.15 | Water | - | - | M27 |
Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
| 1 |
Zeiss #63 HE |
572/25 |
FT 590 |
629/62 |
mCherry, Texas Red |
| 2 |
Zeiss #38 HE |
470/40 |
FT 495 |
525/50 |
EGFP, Alexa Fluor 488 |
| 3 |
Zeiss #47 HE |
436/25 |
FT 455 |
480/40 |
DAPI, Hoechst |
| 4 | mCherry LP | 560/20 | 580LP | 585LP | mCherry, Cy3 |
| 5 |
- |
- | - | - |
- |
| 6 |
- |
- | - | - |
- |
Lasers:
|
Laser |
Wavelength |
Laser Power |
BFP Power* |
Suitable Dyes |
|
405 |
405nm |
- |
- |
DAPI / Hoechst |
|
Argon Ion |
458nm |
- |
- |
CFP |
|
Argon Ion |
488nm |
- |
- |
GFP / A488 |
|
Argon Ion |
514nm |
- |
- |
YFP |
|
561 |
561nm |
- |
- |
mCherry / Cy3 |
|
594 |
594nm |
- |
- |
A594 |
|
633 |
633nm |
- |
- |
Cy5 / A647 |
*BFP = Back Focal Plane of Objective
Computer:
Fujitsu PC - 3GHz Xeon Processor - 32GB RAM - 1GB nVidia Quadro GPU
3TB Raid Array
30" LCD Monitor
Software:
Zeiss Zen 2012 Black
Accessories:
Incubation chamber with CO2 and Temperature control
Andor Dragonfly Spinning Disc Confocal with TIRF
Resource Links:
- Dragonfly Spinning Disc Training Manual
- Training Videos
- Microscope location - Room 6.025b
- Make a Booking

Room 6.025b - Nikon Inverted Microscope Stand with Andor Dragonfly Spinning Disc Scanhead
Inverted spinning disc laser confocal microscope, suited for fast live imaging, 3-dimensional multi-position and large scale tiling. Also has TIRF functionality and incubation.
Features Include:
- Fully motorised X-Y-Z stage
- Piezo Z-drive for rapid Z-stacks
- CO2 and temperature incubation for live cells and tissue
- Tiled imaging
- Multi-position imaging
- TIRF imaging
- Routine time-lapse and ultra-fast burst mode imaging
Transmitted Light: CoolLED White Light LED
Reflected Light: Nikon Intensilight 100W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| Universal LWD | - | - | - | - | - | - | - | - |
Objectives:
| Position | Objective | Mag | N.A. | Imm | W.D. (mm) | Type | R.I. | XY Res (nm) | Arbitrary Brightness |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Plan Apo λ | 4x | 0.2 | Dry | 20 | - | 1.00 | 1488 | 1 |
| 2 | Plan Apo λ | 10x | 0.45 | Dry | 4.0 | DIC II | 1.00 | 662 | 4 |
| 3 | Plan Apo VC | 20x | 0.75 | Dry | 1.0 | DIC II | 1.00 | 397 | 8 |
| 4 | Plan Apo λ | 40x | 0.95 | Dry | 0.16-0.25 | DIC II | 1.00 | 313 | 5 |
| 5 | Apo λ LWD | 40x | 1.15 | Water | 0.59-0.61 | DIC II | 1.33 | 259 | 11 |
| 6 |
Plan Apo λ |
60x |
1.4 |
Oil |
0.13 |
Ph3 DM |
1.515 |
213 | 11 |
*100x TIRF Oil lens is also available (1.49 N.A.)
Laser Lines Available:
405, 445, 488, 514, 561 and 637nm
Eyepiece Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
|---|---|---|---|---|---|
| 1 | DAPI | 436/25 | 455 | 480/40 | ECFP, Cerulean |
| 2 | GFP | 470/40 | 495 | 525/50 | EGFP, Alexa Fluor 488 |
| 3 | TRITC | 500/25 | 515 | 535/30 | EYFP, Citrine |
| 4 | - | - | - | - | - |
| 5 | - | - | - | - | - |
| 6 |
- |
- | - | - | - |
Confocal Fluorescence Dichroic Sets:
|
First Dichroic Set |
Second Dichroic Set | Third Dichroic Set |
|---|---|---|
|
405-13 |
405-13 | 405-13 |
| 488-13 | 445-13 | 488-13 |
| 561-6 | 514-14 | 561-6 |
| 640-16 | 640-16 | 685-13 |
Confocal Emission Filter Wheel Options:
| Position | Camera 1 | Camera 2 |
|---|---|---|
| 1) | 445-514 | TR-442-514-561 |
| 2) | 700-75 | 600-50 |
| 3) | 620-60 | 525-50 |
| 4) | 600-50 | 450-50 |
| 5) | 525-50 | TR-POL-DIC |
| 6) | 450-50 | Blocked |
| 7) | TR-POL-DIC | Blocked |
| 8) | Blocked | Blocked |
Computer:
Fujitsu PC - 3GHz Xeon Processor - 92GB RAM - 8GB nVidia Quadro GPU
4TB Raid Array
30" LCD Monitor
Software:
Fusion and Imaris
Accessories:
Incubation chamber with CO2 and Temperature control
Spinning Disc Confocal - Inverted Cell Observer SD
Resource Links:
- Spinning Disc Confocal Training Manual
- Camera Alignment Manual
- Zeiss Filters
- Training Videos
- Microscope location - Room 6.018
- Make a Booking

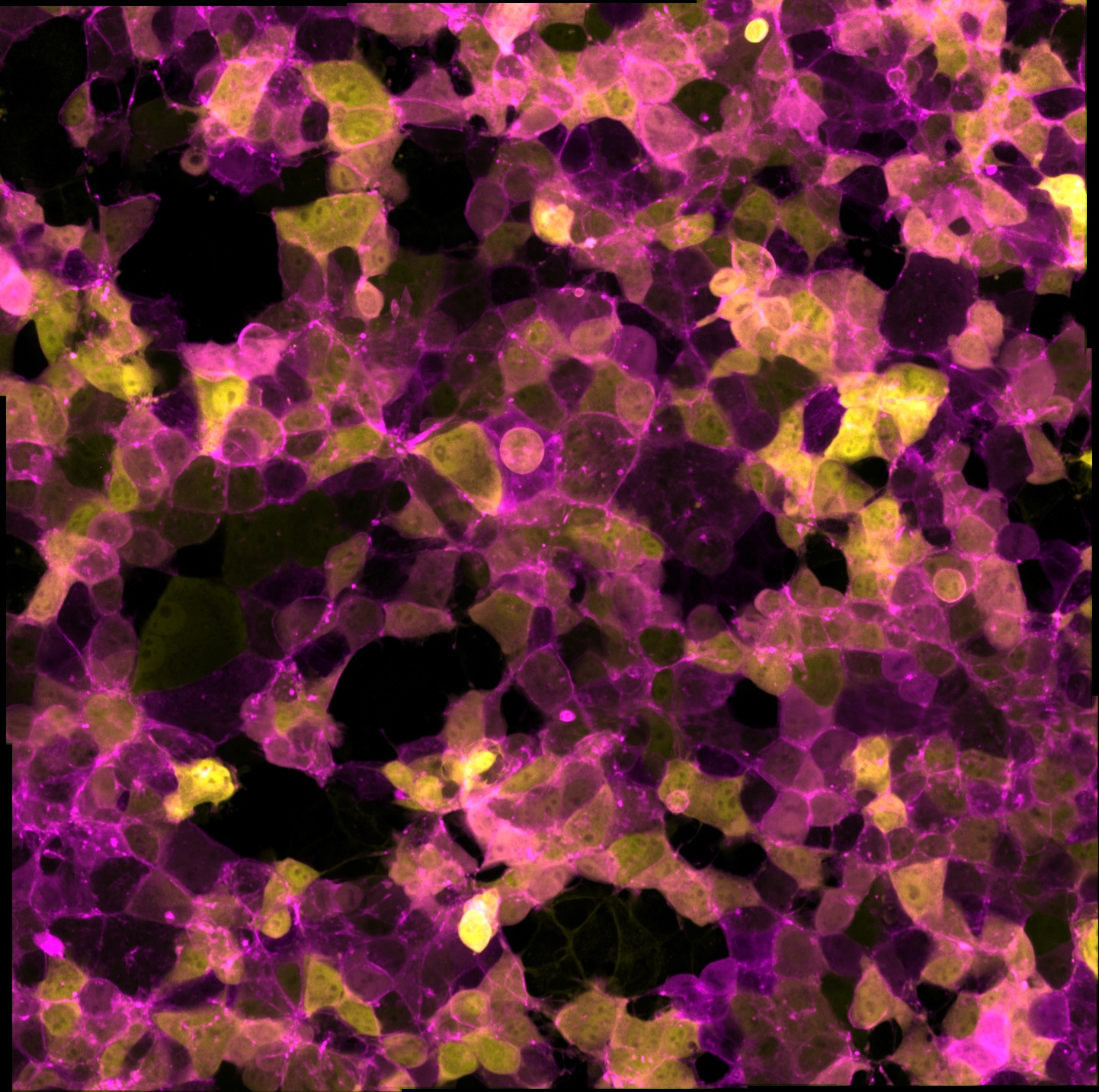
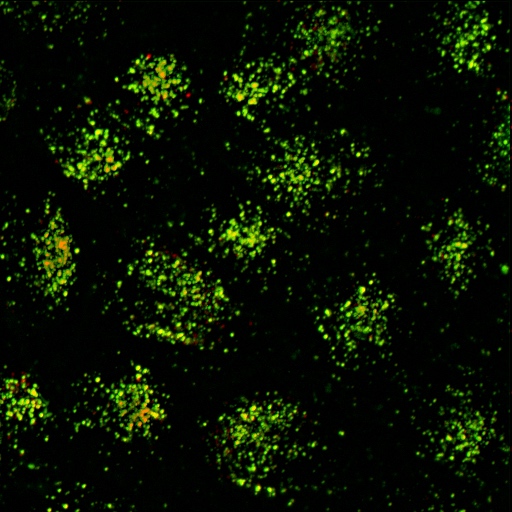
Room 6.018 - Zeiss Axiovert 200 Inverted Microscope Stand with CSU-X1 scanhead
Inverted spinning disc laser confocal microscope Suited for live imaging.
Features Include:
- Fully motorised X-Y-Z stage
- Piezo Z-drive for rapid Z-stacks
- CO2 and temperature incubation for live cells and tissue
- Tiled imaging
- Multi-position imaging
Transmitted Light: Zeiss 100W Halogen Lamp
Reflected Light: Exfo Xcite 120W White Light Lamp
Condensor:
| Name | N.A. | W.D. mm | Position 1 | Position 2 | Position 3 | Position 4 | Position 5 | Position 6 |
| Universal LWD | 0.45 | 54 | Open | PhL | Ph1 | Ph2 | DIC I | DIC II |
Objectives:
| Position | Objective | Magnification | N.A. | Immersion | W.D. (mm) | Type | Type | Thread |
| 1 | Plan Apochromat | 40x | 0.95 | Dry | - | DIC III | - | M27 |
| 2 | C Apo | 40x | 1.2 | Water | - | DIC III | M27 | |
| 3 | LCI PlanN | 63x | 1.3 | Water | - | DIC III | M27 | |
| 4 | Plan Apochromat | 10x | 0.45 | Dry | - | DIC II | M27 | |
| 5 | Plan Apochromat | 20x | 0.8 | Dry | - | DIC II | M27 | |
| 6 |
- |
- |
- |
- |
- |
- |
- |
Eyepiece Fluorescent Filter Sets:
| Position | Name | Excitation | Dichroic | Emission | Suitable Dyes |
| 1 | CFP (47HE) | 436/25 | 455 | 480/40 | ECFP, Cerulean |
| 2 | GFP (38HE) | 470/40 | 495 | 525/50 | EGFP, Alexa Fluor 488 |
| 3 | YFP (46HE) | 500/25 | 515 | 535/30 | EYFP, Citrine |
| 4 | RFP (63HE) | 572/25 | 590 | 629/62 | mCherry, Cy3 |
| 5 | - | - | - | - | - |
| 6 |
- |
- | - | - | - |
Confocal Fluorescence Filter Sets:
|
First Dichroic |
Second Dichroic | Camera1 | Camera2 |
|
405/488/561 |
Empty | 485/30 | 562/45 |
| 457/514/647 | 510LP | 535/30 | 630/98 |
| 405/488/561/647 | 560LP | - | - |
Computer:
Fujitsu PC - 3GHz Xeon Processor - 32GB RAM - 1GB nVidia Quadro GPU
3TB Raid Array
30" LCD Monitor
Software:
Zeiss Zen 2012 Blue
Accessories:
Incubation chamber with CO2 and Temperature control
