Note for users
For UQ Staff and Students, please click here to view our Hourly rates, book equipment or request training.
For external users, please contact the IMB Microscopy team before requesting an account. You may view our hourly rates at the link above
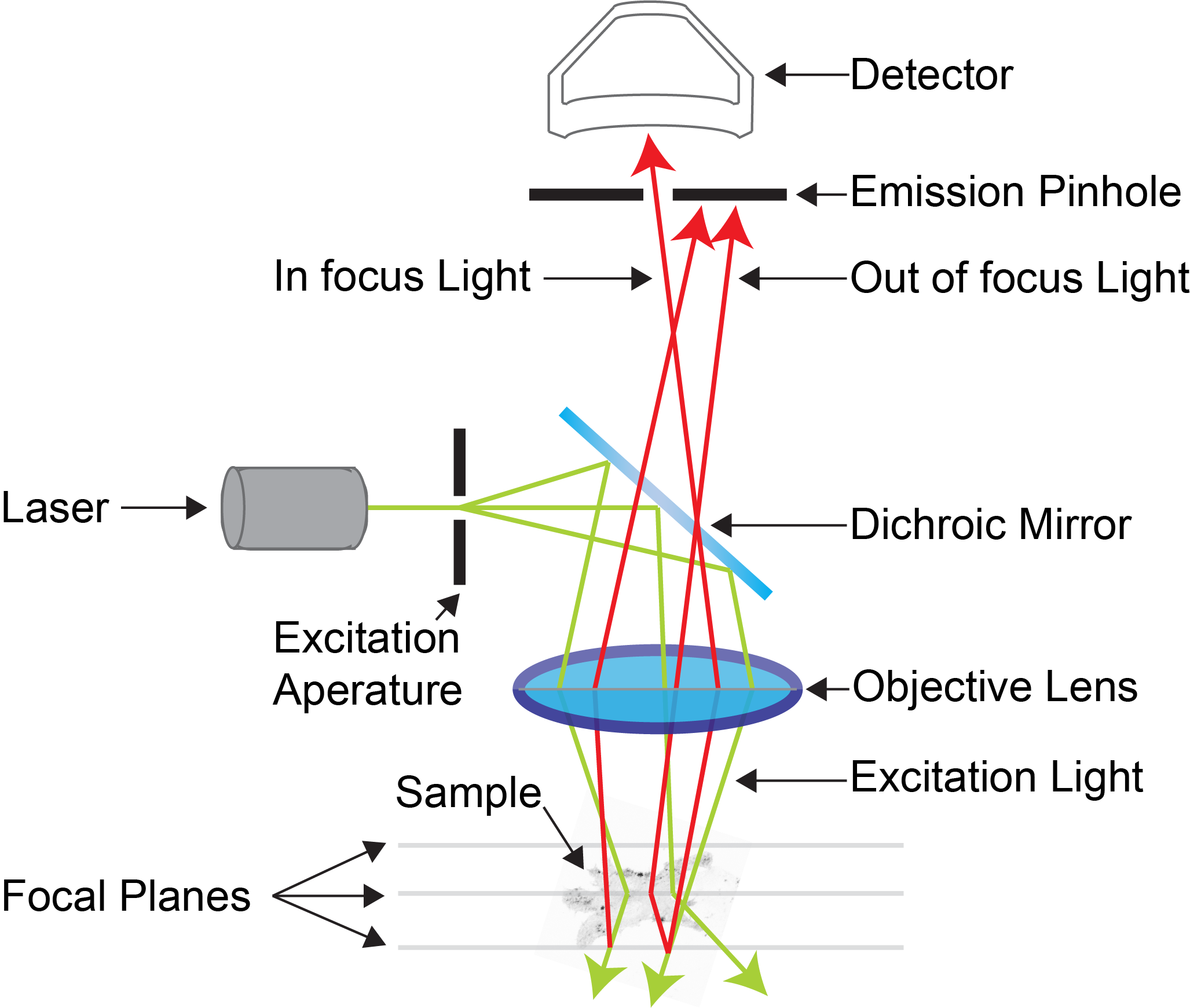
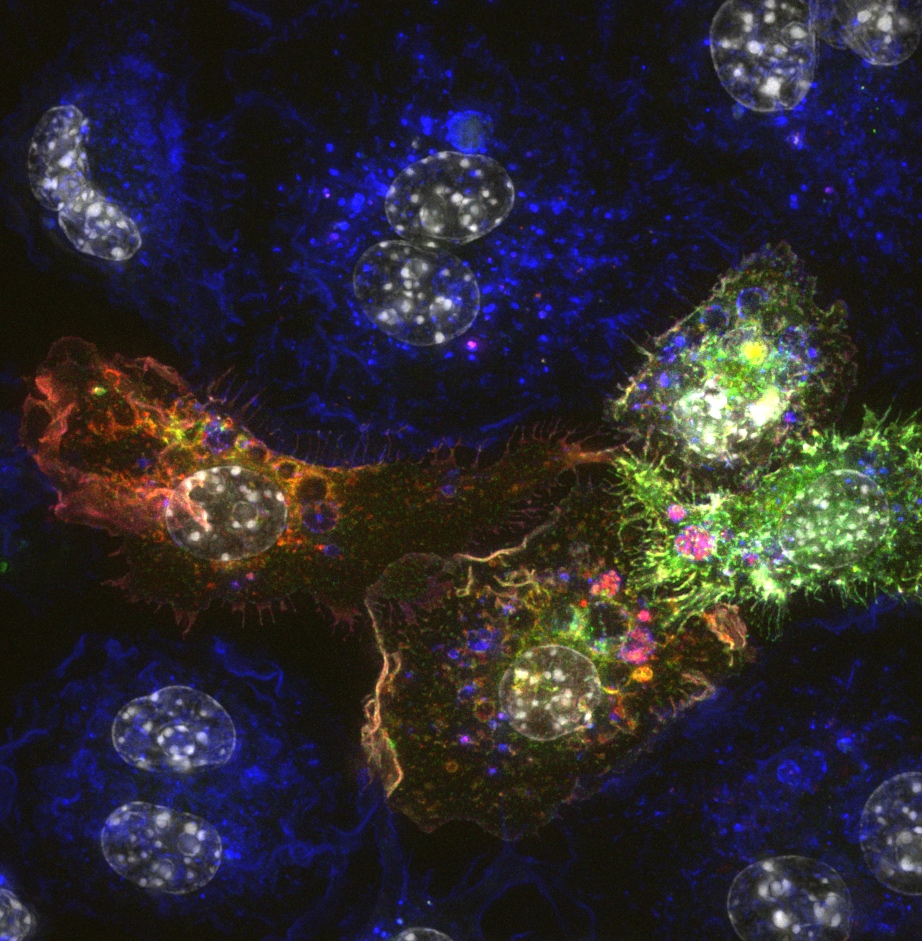
Confocal microscopy is an advanced light microscopy method which utilises a ‘pinhole’ to eliminate out of focus light and is suitable for both live and fixed cells and tissues. The advantage of a confocal microscope over conventional wide-field microscopes is that discrete optical sections can be collected while eliminating the out of focus light above and below the current plane of focus. Because of this, high powered lasers are used to illuminate the sample in order to collect enough light only from the desired focal plane.
Focused beams of laser light are scanned across the sample (Laser Scanning Microscopy, LSM) and light only from the desired focal plane is allowed to enter the detector. Depending on the fluorophores in your sample, and the number of detectors available, multiple fluorophores can be excited and detected simultaneously. Traditional detectors are photo-multiplier tubes, however more recent Gallium Arsenide Phosphide (GaAsP) detectors have been developed to increase sensitivity (Confocal 2 and 4, Airyscan; Confocal 5, BiG detectors). Leica have also developed their own higher sensitivity detector, known as a HyD - a hybrid detector combining the best properties of traditional PMTs and the highly sensitive avalanch photo-diode detectors (Confocal 1, HyD).
These microscopes provide excellent axial resolution, and very good signal to noise sampling, however this often comes with a sacrifice in temporal resolution due to the slow nature of scanning pixel-by-pixel across the image during capture. Some systems overcome this speed issue with either faster scanners (Leica SP8 - Confocal 1 uses an 8 kHz resonant scanner) or with multiple pinholes (Andor Dragonfly Spinning Disc Confocal and Yokogawa CSU-X1) solving this speed limitation in unique ways.
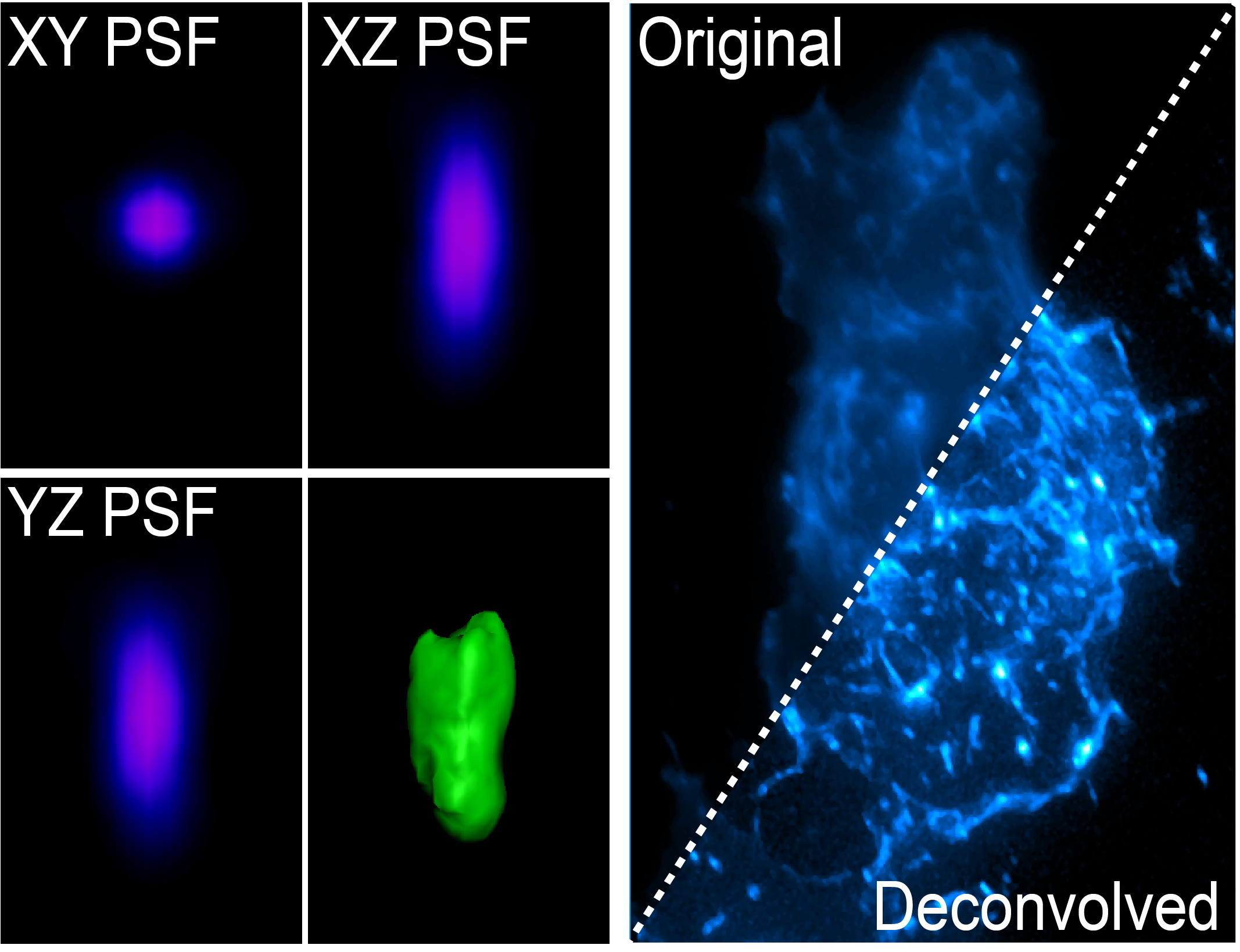 Wide-field microscopy involves illumination of the whole sample, and relies on the optical characteristics of the imaging system to capture a single focal plane. The limitation of these kinds of imaging systems is that often, out of focus light (from differing sample planes) are captured in your images, resulting in image-blur. This image blur will lower your signal to noise, which is the contrast of your images. One way to overcome this is by using specialised optics with very narrow depths of field (High NA objectives) coupled with computational algorithms that can correct for out of focus light, this process is called deconvolution.
Wide-field microscopy involves illumination of the whole sample, and relies on the optical characteristics of the imaging system to capture a single focal plane. The limitation of these kinds of imaging systems is that often, out of focus light (from differing sample planes) are captured in your images, resulting in image-blur. This image blur will lower your signal to noise, which is the contrast of your images. One way to overcome this is by using specialised optics with very narrow depths of field (High NA objectives) coupled with computational algorithms that can correct for out of focus light, this process is called deconvolution.
The light coming from your sample scatters (diffraction) in all directions when illuminated, and this can be affected by coverslip thickness, sample mounting medium and the many components of the microscope between your sample and the camera. The way that the light diffracts can be characterised by the point spread function (PSF) whereby a single point of light of known size (fluorescent bead) is captured in xyz and shows the scattering behaviour of light in all dimensions for that particular optical system. The PSF is then used to reverse the effects of out of focus light by correcting for these known distortions in all dimensions.
The PSF can be generated for your particular sample using a fluorescent bead that is smaller than the smallest resolvable structure by the particular imaging system, or it can be theoretically generated using known algorithms. Two main deconvolution algorithms exist, the Richardson-Lucy and Wiener deconvolution algorithms. Deconvolution is an iterative process which is cycled on your images. Deconvolution is a post processing method, and is available either on the microscope (Deltavision, Nikon Deconvolution Microscope) or via software (Analysis Computers, Microvolution, AutoQuant).
The Imaging facility currently has 4x dedicated, physical workstation computers as well as a number of virtualised machines available for image processing and visualisation of your microscopy data.
Analysis virtual machines are high-powered, graphics accelerated computers available to all users of the imaging facility to access, either onsite at the Queensland Bioscience Precinct, or remotely via VPN. These machines are installed with multiple different software packages including all of the relevant vender software (Zen, LAS X, NIS Elements Viewer), deconvolution packages such as Huygens HyVolution and Microvolution, as well as advanced visualisation and analysis tools including Imaris 9, and FIJI/ImageJ.
These virtual machines are Big Data Ready! This means they have a large allocation of RAM (1Tb or more), 3 x Volta V100 16Gb GPU processors each, high-speed network connections, a large dedicated scratch workspace (200Tb) and the latest visualisation software, including Imaris 9 and Arivis-4D. For large datasets (Spinning Disc Confocal, Lattice Lightsheet Microscope, Nikon deconvolution) we recommend Imaris 9 for visualisation and quantification.
Access to these machines within IMB will require Microsoft Remote Desktop Viewer, while access from outside of IMB will also require Cisco Anyconnect software in addition to the RDP viewer. Bookings for these machines are available via https://au.ppms.info/uq-imb/.