Deconvolution Microscopes
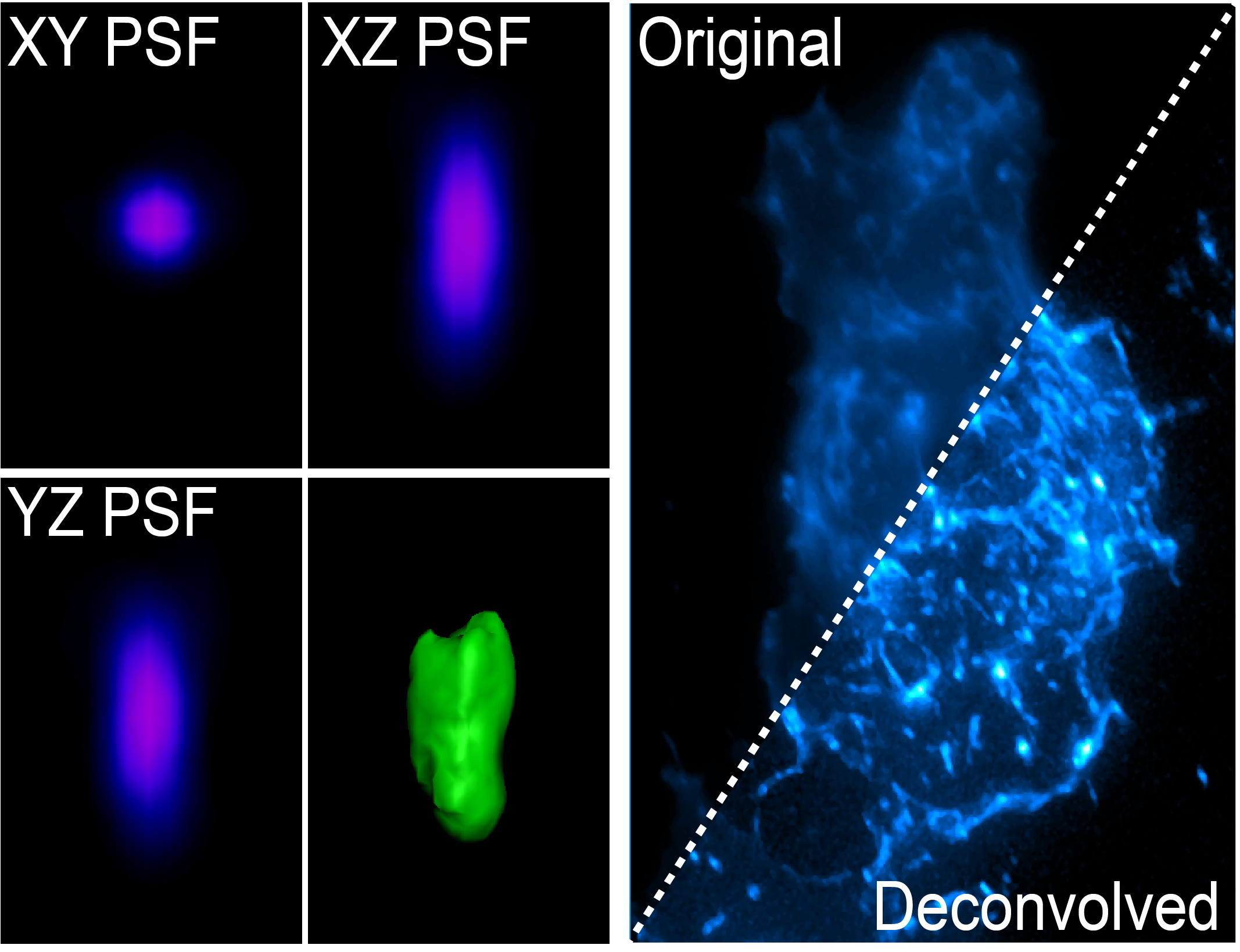 Wide-field microscopy involves illumination of the whole sample, and relies on the optical characteristics of the imaging system to capture a single focal plane. The limitation of these kinds of imaging systems is that often, out of focus light (from differing sample planes) are captured in your images, resulting in image-blur. This image blur will lower your signal to noise, which is the contrast of your images. One way to overcome this is by using specialised optics with very narrow depths of field (High NA objectives) coupled with computational algorithms that can correct for out of focus light, this process is called deconvolution.
Wide-field microscopy involves illumination of the whole sample, and relies on the optical characteristics of the imaging system to capture a single focal plane. The limitation of these kinds of imaging systems is that often, out of focus light (from differing sample planes) are captured in your images, resulting in image-blur. This image blur will lower your signal to noise, which is the contrast of your images. One way to overcome this is by using specialised optics with very narrow depths of field (High NA objectives) coupled with computational algorithms that can correct for out of focus light, this process is called deconvolution.
The light coming from your sample scatters (diffraction) in all directions when illuminated, and this can be affected by coverslip thickness, sample mounting medium and the many components of the microscope between your sample and the camera. The way that the light diffracts can be characterised by the point spread function (PSF) whereby a single point of light of known size (fluorescent bead) is captured in xyz and shows the scattering behaviour of light in all dimensions for that particular optical system. The PSF is then used to reverse the effects of out of focus light by correcting for these known distortions in all dimensions.
The PSF can be generated for your particular sample using a fluorescent bead that is smaller than the smallest resolvable structure by the particular imaging system, or it can be theoretically generated using known algorithms. Two main deconvolution algorithms exist, the Richardson-Lucy and Wiener deconvolution algorithms. Deconvolution is an iterative process which is cycled on your images. Deconvolution is a post processing method, and is available either on the microscope (Deltavision, Nikon Deconvolution Microscope) or via software (Analysis Computers, Microvolution, AutoQuant).