Widefield Microscopes
Widefield microscopy is a commonly used method for viewing dynamic and topographical information which encompasses many different specialities including brightfield, differential interference contrast (DIC), phase-contrast and epifluorescence. In a widefield microscope, the entire sample is illuminated and the sample is optically sectioned purely via the objective lens. These systems are typically faster than confocal; however, samples are hit with more light and often have a lower signal: noise ratio.
Most widefield microscopes have two light sources, a halogen bulb for brightfield, and a much brighter fluorescence light source (usually a mercury lamp). In the example upright widefield microscope, the brightfield lamp is housed in the base of the microscope and passes through a field stop and condenser before hitting the sample. In comparison, the fluorescence light source is at the rear of the microscope and passes through a filter cube before being focused by the objective onto the sample.
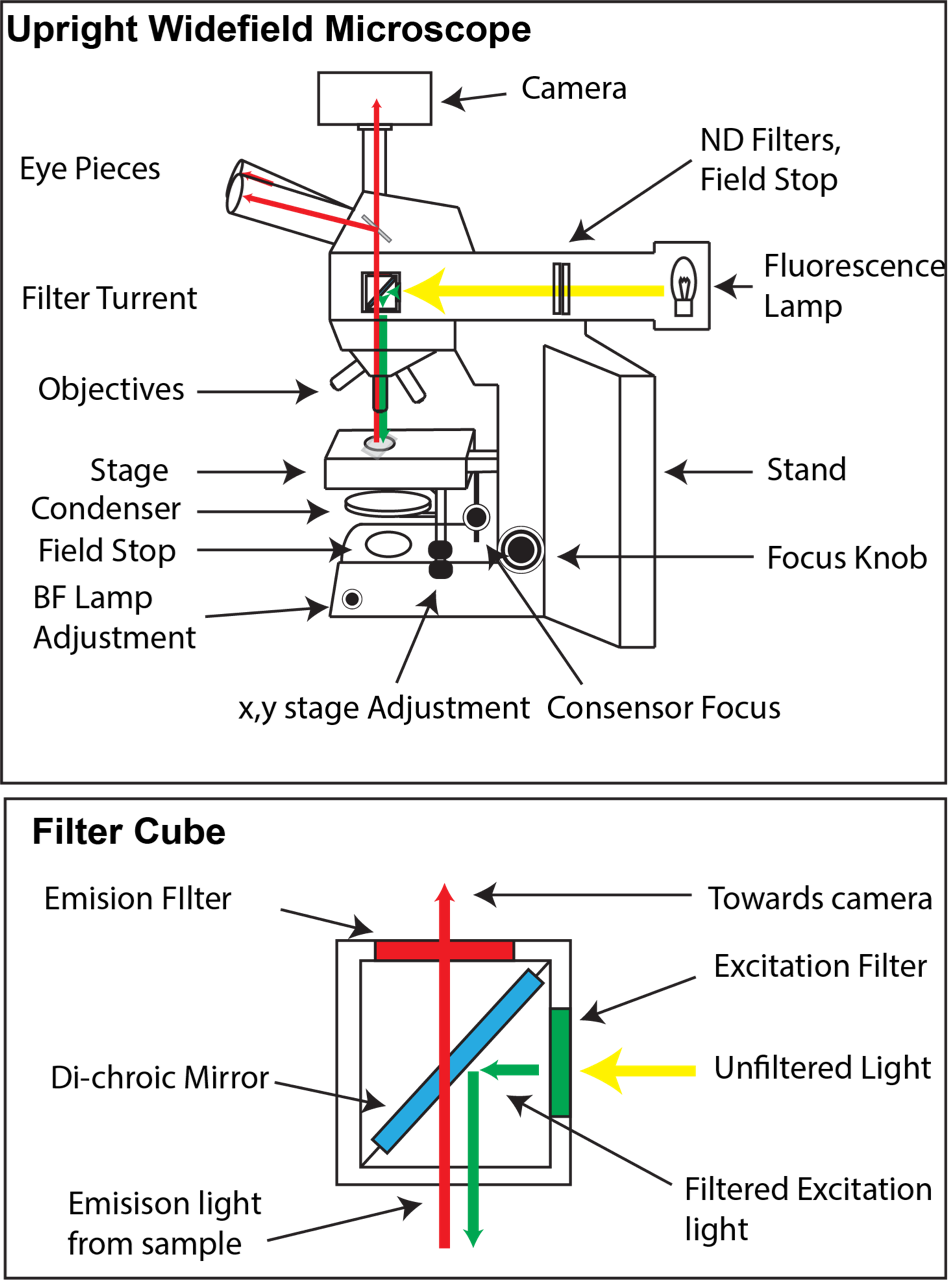
In fluorescence microscopy, light of a particular wavelength is used to excite a molecule which in turn emits light at a different wavelength before being detected via a camera. Filter cubes contain two glass filters and a special mirror called a di-chroic. Unfiltered (white) light first enters the cube via an excitation filter (this only allows light from a narrow range of the spectrum through). The di-chroic mirror reflects particular wavelengths of light, while allowing others to pass through. Here the green excitation light is reflected, sending it down to the sample. Emission light (longer wavelength than excitation light) that comes back from the sample can pass freely through the di-chroic and is then filtered with the emission filter. Typically our microscopes hold 4-6 filter cubes often suited for a wide variety of dyes and fluorophore combinations such as DAPI, CFP, GFP/Alexa488, YFP, RFP/mCherry, or Cy5/Alexa647.
 How does a widefield microscope work?
How does a widefield microscope work?
Most widefield microscopes have two light sources, a halogen bulb for brightfield, and a much brighter fluorescence light source (usually a mercury lamp). In the example upright widefield microscope, the brightfield lamp is housed in the base of the microscope and passes through a field stop and condenser before hitting the sample. In comparison, the fluorescence light source is at the rear of the microscope and passes through a filter cube before being focused by the objective onto the sample.
In fluorescence microscopy, light of a particular wavelength is used to excite a molecule which in turn emits light at a different wavelength before being detected via a camera. Filter cubes contain two glass filters and a special mirror called a di-chroic. Unfiltered (white) light first enters the cube via an excitation filter (this only allows light from a narrow range of the spectrum through). The di-chroic mirror reflects particular wavelengths of light, while allowing others to pass through. Here the green excitation light is reflected, sending it down to the sample. Emission light (longer wavelength than excitation light) that comes back from the sample can pass freely through the di-chroic and is then filtered with the emission filter. Typically our microscopes hold 4-6 filter cubes often suited for a wide variety of dyes and fluorophore combinations such as DAPI, CFP, GFP/Alexa488, YFP, RFP/mCherry, or Cy5/Alexa647.
Contacts
Dr James Springfield
Microscopy Facility Manager
+61 7 334 62390
j.springfield@imb.uq.edu.au
Dr Nicholas Condon
CZI Imaging Scientist
+61 7 334 62042
n.condon@imb.uq.edu.au
Dr Mahdie Mollazade
Microscopy Officer
+61 7 334 62042
m.mollazade@uq.edu.au
Mailing address
Advanced Microscopy Facility
Institute for Molecular Bioscience
Level 6N, 306 Carmody Road,
Building 80
University of Queensland
4072, St Lucia,
Queensland, Australia
