Single Plane Illumination Techniques (Light-sheet Microscopy)
Sections:
- Introduction
- Optical Sectioning
- Sample Mounting
- 2 Photon Light-sheet
- Lattice Light-sheet Microscopy
- IMB Lattice Light-sheet Microscopy
IMB Systems: openSPIM
Reference: https://www.microscopyu.com/techniques/light-sheet/light-sheet-fluorescence-microscopy
Introduction
The Excerpts below are taken from: https://en.wikipedia.org/wiki/Light_sheet_fluorescence_microscopy
Light sheet fluorescence microscopy (LSFM) is a fluorescence microscopy technique with an intermediate-to-high[1] optical resolution, but good optical sectioning capabilities and high speed. In contrast to epifluorescence microscopy only a thin slice (usually a few hundred nanometers to a few micrometers) of the sample is illuminated perpendicularly to the direction of observation. For illumination, a laser light-sheet is used, i.e. a laser beam which is focused only in one direction (e.g. using a cylindrical lens). A second method uses a circular beam scanned in one direction to create the lightsheet. As only the actually observed section is illuminated, this method reduces the photodamage and stress induced on a living sample. Also the good optical sectioning capability reduces the background signal and thus creates images with higher contrast, comparable to confocal microscopy. Because LSFM scans samples by using a plane of light instead of a point (as in confocal microscopy), it can acquire images at speeds 100 to 1000 times faster than those offered by point-scanning methods.
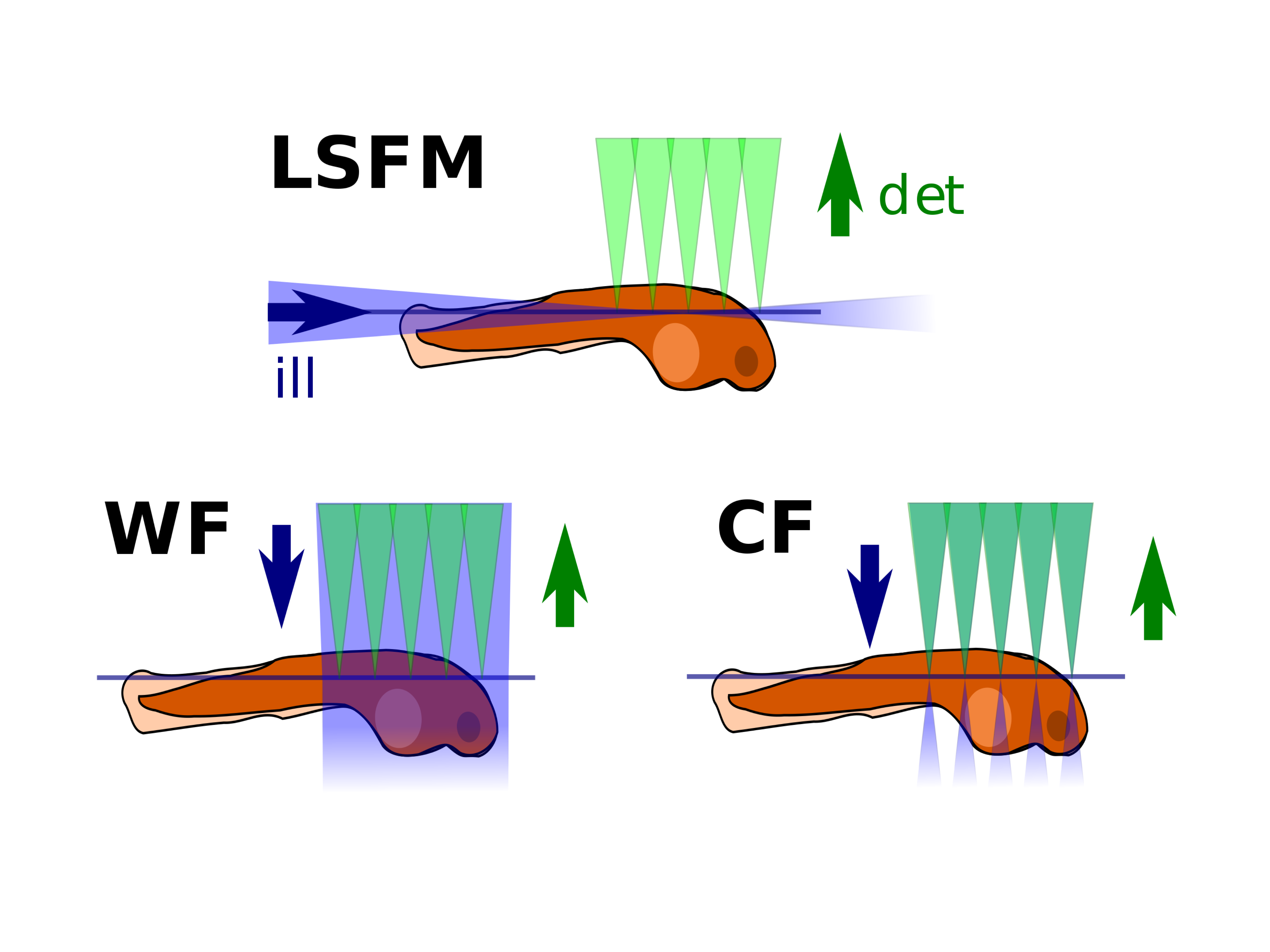
Comparison of different microscopy illumination modalities (LSFM: lightsheet fluorescence microscopy, WF: widefield microscopy, CF: confocal microscopy). LSFM combines good z-sectioning (as confocal) and only illuminates the observed plane
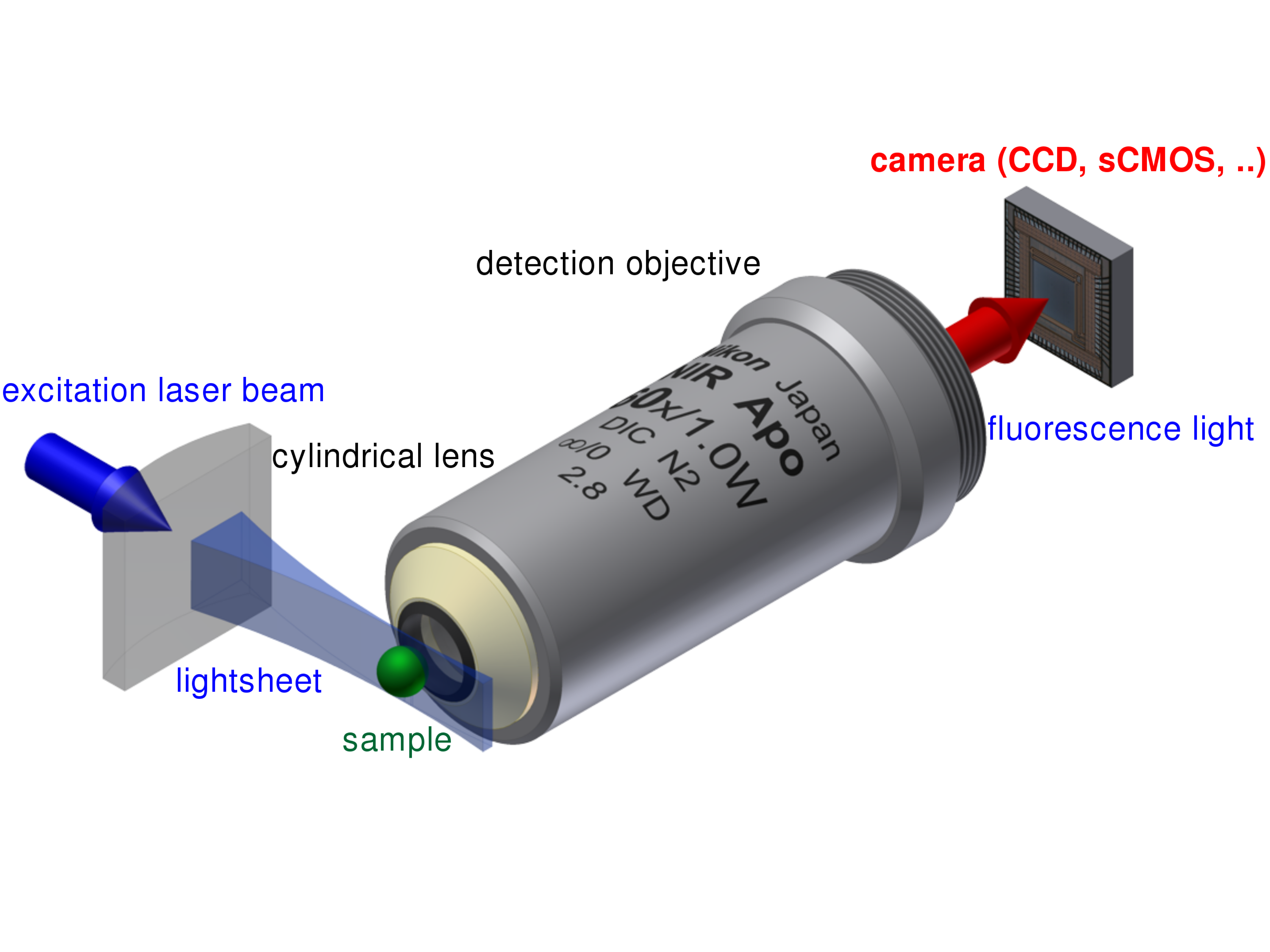
The principle setup of a light sheet fluorescence microscope.
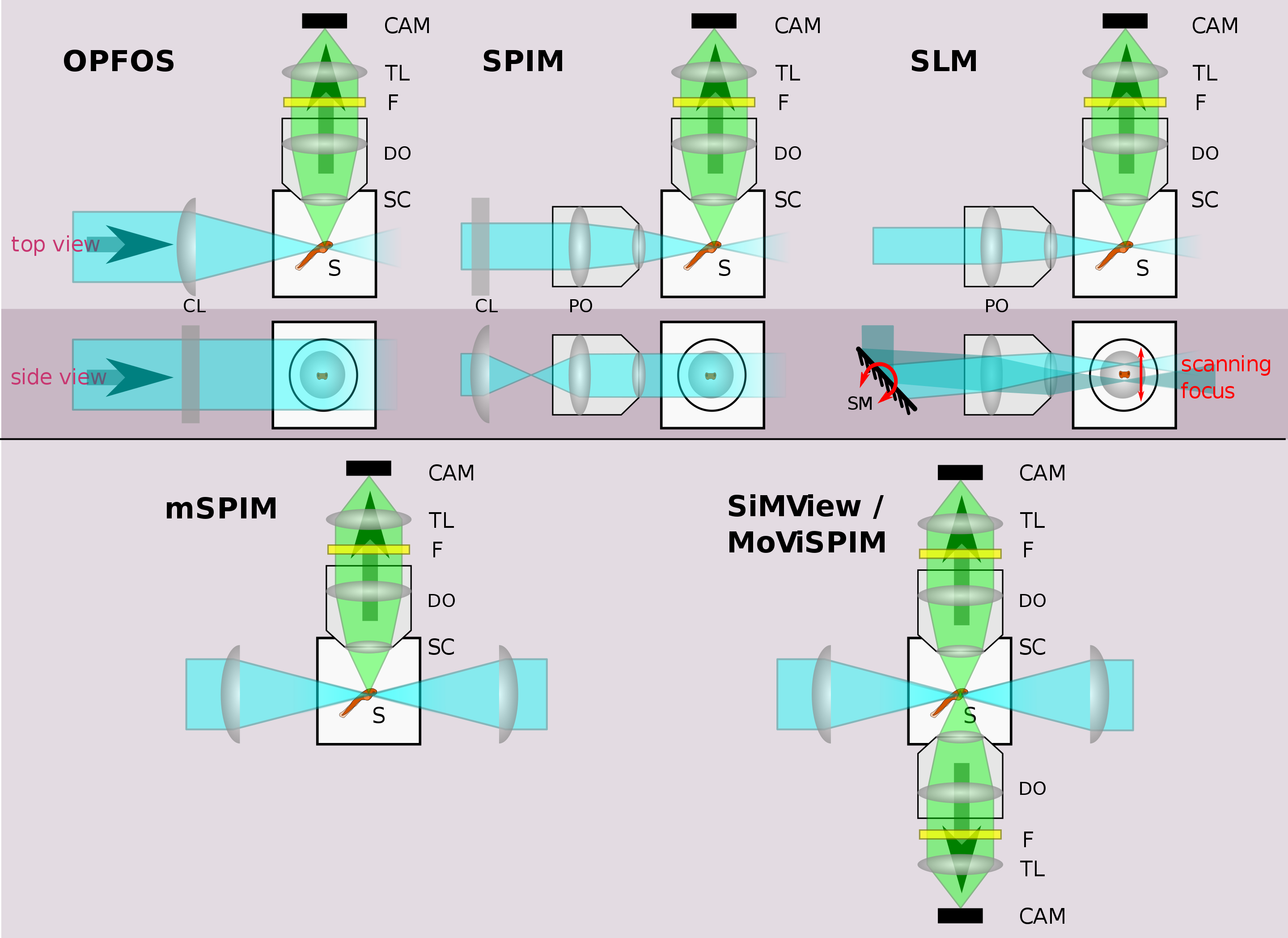
Illustration of different LSFM implementations. See text for details. Legend: CAM=camera, TL=tube lens, F=filter, DO=detection objective, S=sample, SC=sample chamber, PO=projection objective, CL=cylindrical lens, SM=scanning mirror
Optical Sectioning
Since LSFM inherently produces a high contrast single play image, it may be utilised to image a single 2D plane at extremely high temporal resolution, limited only by the sample brightness, sensitivity and maximum frame rate of the camera, with typical speeds of 50 to 100fps at 4Mp for a sCMOS camera. However to produce a 3D volume either the sample must be moved through the lightsheet (which is centred on the focal plane of the detection objective) or the lighsheet must be moved synchronously with the focal plane of the detection objective. Both methods are utilised in commercial systems and there are many Pro's and Cons to each method, including disturbance of the sample and loss of detection resolution as two of the main Cons. However the end result may produce a high contrast 3D volume anywhere from one volume a minute up to hundreds of volumes a second.
Sample Mounting
The separation of the illumination and detection beampaths in LSFM (except in oblique plane microscopy) creates a need for specialized sample mounting methods. To date most LSFMs are built in such a way that the illumination and detection beampath lie in a horizontal plane (see illustrations above), thus the sample is usually hanging from the top into the sample chamber or is resting on a vertical support inside the sample chamber. Several methods have been developed to mount all sorts of samples:
- Fixed (and potentially also cleared) samples can be glued to a simple support or holder and can stay in their fixing solution during imaging.
- Larger living organisms are usually sedated and mounted in a soft gel cylinder that is extruded from a (glass or plastic) capillary hanging from above into the sample chamber.
- Adherent cells can be grown on small glass plates that are hanging in the sample chamber.
- Plants can be grown in clear gels containing a growth medium. The gels are cut away at the position of imaging, so they do not reduce the lightsheet and image quality by scattering and absorption.[28]
- Liquid samples (e.g. for fluorescence correlation spectroscopy) can be mounted in small bags made of thin plastic foil matching the refractive index of the surrounding immersion medium in the sample chamber.[20]
Some LSFMs have been developed where the sample is mounted as in standard microscopy (e.g. cells grow horizontally on the bottom of a petri dish) and the excitation and detection optics are constructed in an upright plane from above. This also allows combining a LSFM with a standard inverted microscope and avoids the requirement for specialized sample mounting procedures.[19][29][30][31]
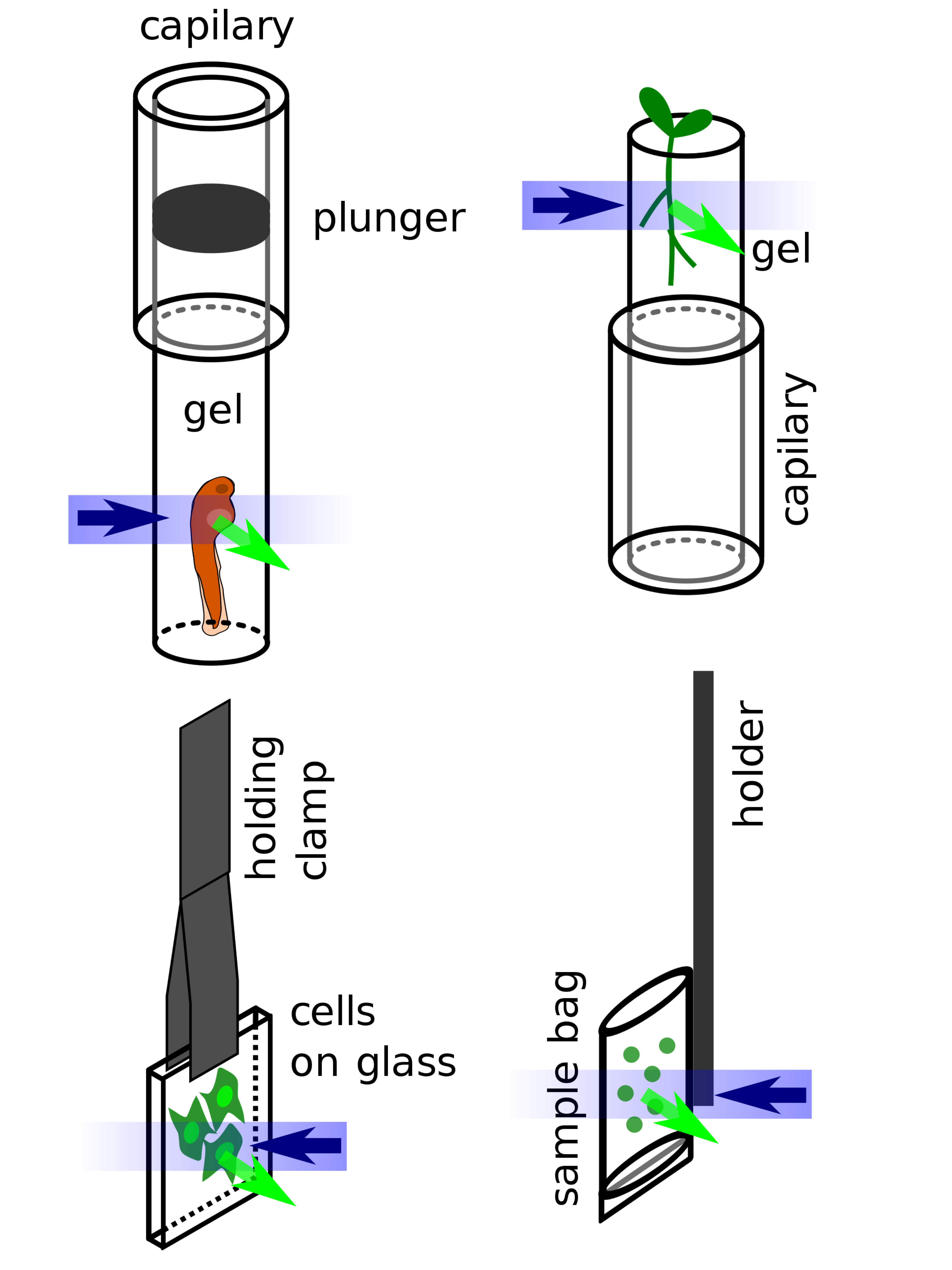
Different types of sample mounting for LSFM: embryo embedded in hanging gel cylinder, plant growing in supported gel cylinder, adherent cells on glass, liquid sample in a sample bag
Light Sheet Tutorial
Introduction to Light Sheet Microscopy
Microscopy: Light Sheet Sectioning (Ernst Stelzer)
Zebrafish development Lightsheet microscopy 3 days
2Photon Lightsheet Microscopy
LSFM has also been combined with two-photon (2P) excitation, which improves the penetration into thick and scattering samples.[17] Use of 2P excitation in near-infrared wavelengths has been used to replace 1P excitation in blue-visible wavelengths in brain imaging experiments involving response to visual stimuli.[18]
UQ QBI Microscopy: https://qbi.uq.edu.au/research/facilities/micro-facility/instruments/2p-lightsheet
2-photon lightsheet calcium imaging in larval zebrafish
Whole-brain imaging of larval zebrafish
Comparison of 1 photon and 2 photon excitation using custom built light sheet microscope
Lattice Light-sheet Microscopy
Background
The Lattice Light Sheet Microscope (LLSM) is a custom microscope developed by Nobel Laureate Eric Betzig and released in late 2014 (Chen et al., 2014). Built upon light sheet imaging technologies, LLSM has improved sample penetration and allows for very high speed, 4-dimensional image acquisition (Chen et al., 2014). Importantly and uniquely, LLSM induces almost no photobleaching or photodamage, which means live samples can be imaged for very long periods of time (hours or even days).
In comparison to conventional laser microscopes, light sheet microscopes generally illuminate a plane (sheet) of laser light through the sample which is detected via a lens positioned perpendicular to the illumination sheet (Santi, 2011). Initially light sheet microscopes utilised cylindrical lenses to form a relatively thick sheet of light (>3 mm) that does not uniformly illuminate across a 10 mm focal point, meaning subcellular structures and dynamics cannot be resolved (Huisken et al., 2004). The LLSM overcomes this resolution issue through the generation of a structured light pattern (lattice) that allows for subcellular resolution (Heddleston and Chew, 2016; Huisken et al., 2004).
The animation below depicts the objective orientation of the LLSM whereby the light-sheet extends from the Illumination objective through the sample at an angle of 31.8 degrees. The detection objective captures the single illuminated plane and projects it onto one of the two cameras.
Lattice Light-sheet Objective Orientation Overview
The Lattice Light-sheet microscope was developed in 2014 by Eric Betzig while working at Janelia Research Campus. The original design was published in Science and is available here.
Lattice Light-Sheet Sample Movies
IMB System Highlights
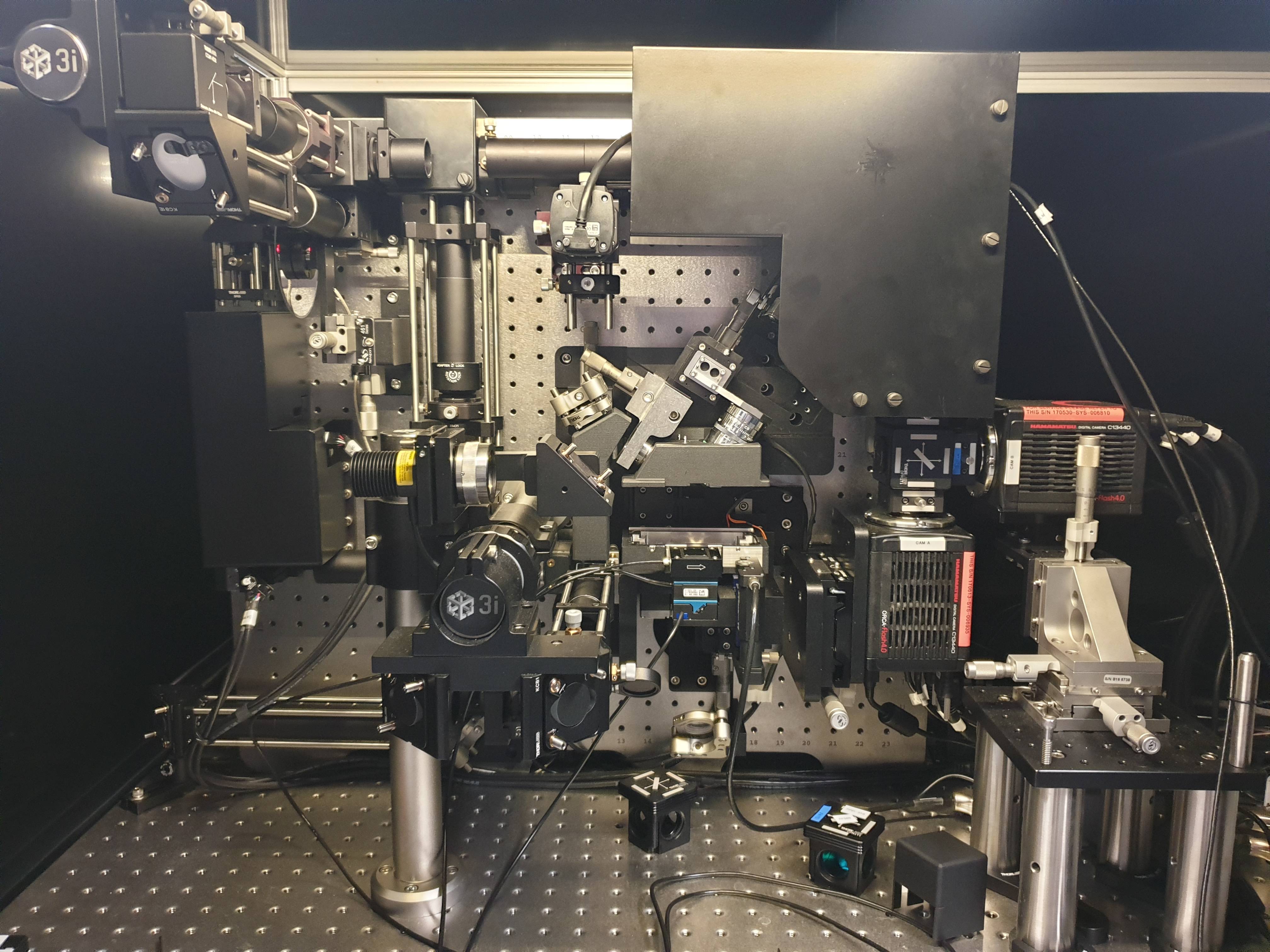
The system housed at the IMB is made by the commercial vendor 3i and is the current Version 2.0.
Key System Features:
- Very High spatial resolution (230 nm, 230 nm, 300 nm, xyz)
- Very High Temporal resolution (100 slices / second)
- Very low photo-toxicity
- Opportunity to generate very rare data (very few working systems world-wide)
- 4 Individual lasers (440 nm (CFP), 488 nm (GFP), 560 nm (RFP), 640 nm (Far Red))
- Dual 4MP Orca Flash 4.0 sCMOS cameras
Key Sample Prep Info:
- This microscope takes 5 mm coverslips (thickness is not important) mounted on the holder shown below.
- The stage can be moved within a 3 x 3 mm region from the centre of the coverslip
- Samples must be adhered to the coverslip (for non-single cell imaging please consult with facility staff)
- Samples are imaged with water dipping lens’ with your sample in a 8 mL water bath (take this into account if your require stimulants/drugs in your imaging media)
- The stage/water-bath is heated, however CO2 is not available while imaging.
- The LLSM room has an incubator set to 37 oC 5% CO2.
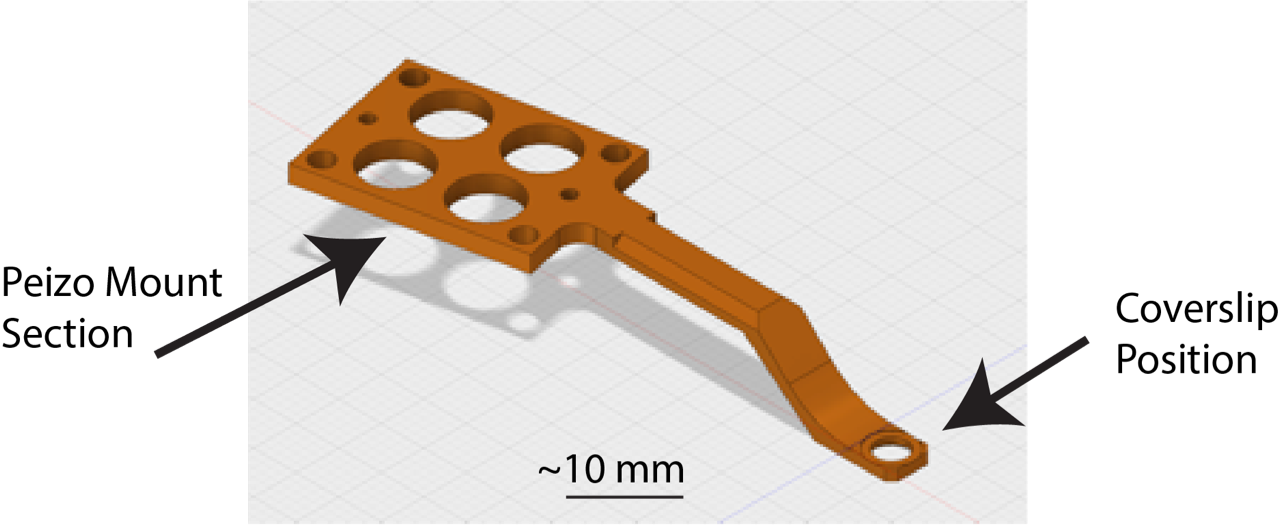
Lattice light-sheet microscopy
Videos from the original Science Paper.
More information on light-sheet microscopes can be found here: https://www.microscopyu.com/techniques/light-sheet/light-sheet-fluorescence-microscopy
