Confocal Techniques
Introduction
Recommended Reading: Tutorial- guidance for quantitative confocal microscopy.pdf
Confocal microscopy is an advanced light microscopy method which utilises a ‘pinhole’ to eliminate out of focus light and is suitable for both live and fixed cells and tissues. The advantage of a confocal microscope over conventional wide-field microscopes is that discrete optical sections can be collected while eliminating the out of focus light above and below the current plane of focus. Because of this, high powered lasers are used to illuminate the sample in order to collect enough light only from the desired focal plane.
Microscopy: Optical Sectioning and Confocal Microscopy (Kurt Thorn)
Optical Sectioning
The benefit of using a confocal is found in its ability to 'throw away' out-of-focus light with the pinhole. This means that 3D objects such as cells, or organisms can be imaged as serial optical sections which can then be recombined to generate 3D reconstructions.
In the diagram below of an example confocal light path we can see the fluorescence coming from the sample that is either above or below the focal plane will arrive spatially at different point which is excluded by the closed down pinhole. Only light that emanated from the focal plane of interest is allowed through to the detector.
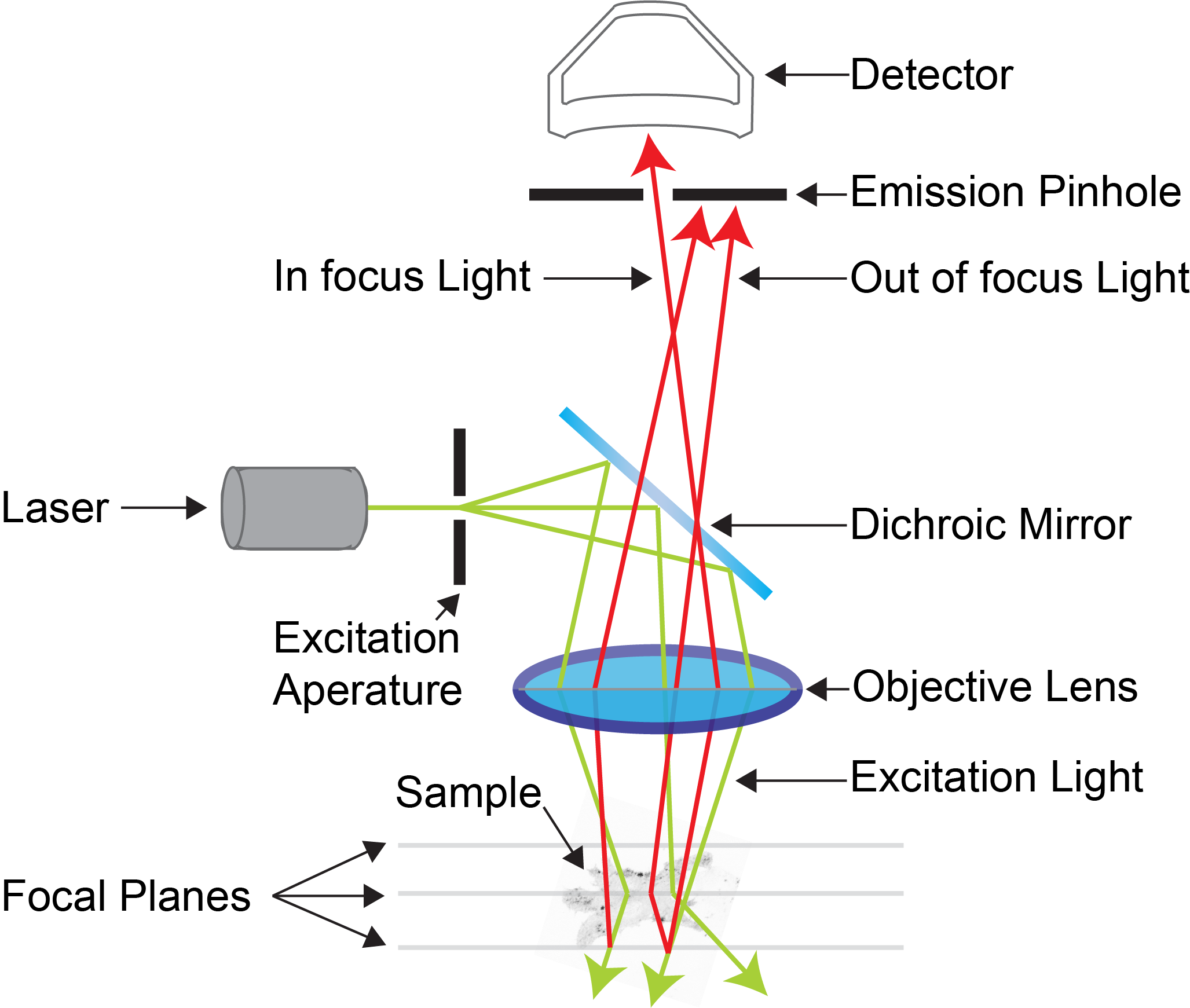
Example confocal light path showing the effect of the emission pinhole on blocking out-of-focus light entering the detector. (Adapted from MicroscopyU)
Importantly on the illumination side, whether you are using a widefield system or a confocal, the sample is still illuminated all the way through axially. The pinhole removes this, whereas on a widefield system all out-of-focus light is still captured. The below two figure represent both widefield and confocal examples (for both illumination, and detection).
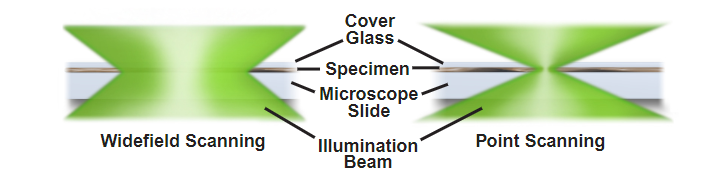
Widefield vs Point scanning of specimens.
Source: MicroscopyU
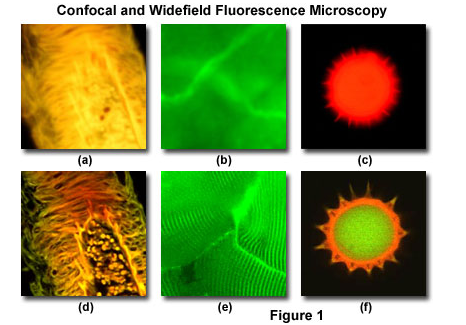
Comparison between widefield and single optical sections from a confocal of the same samples.
Source: Olympus Life Sciences
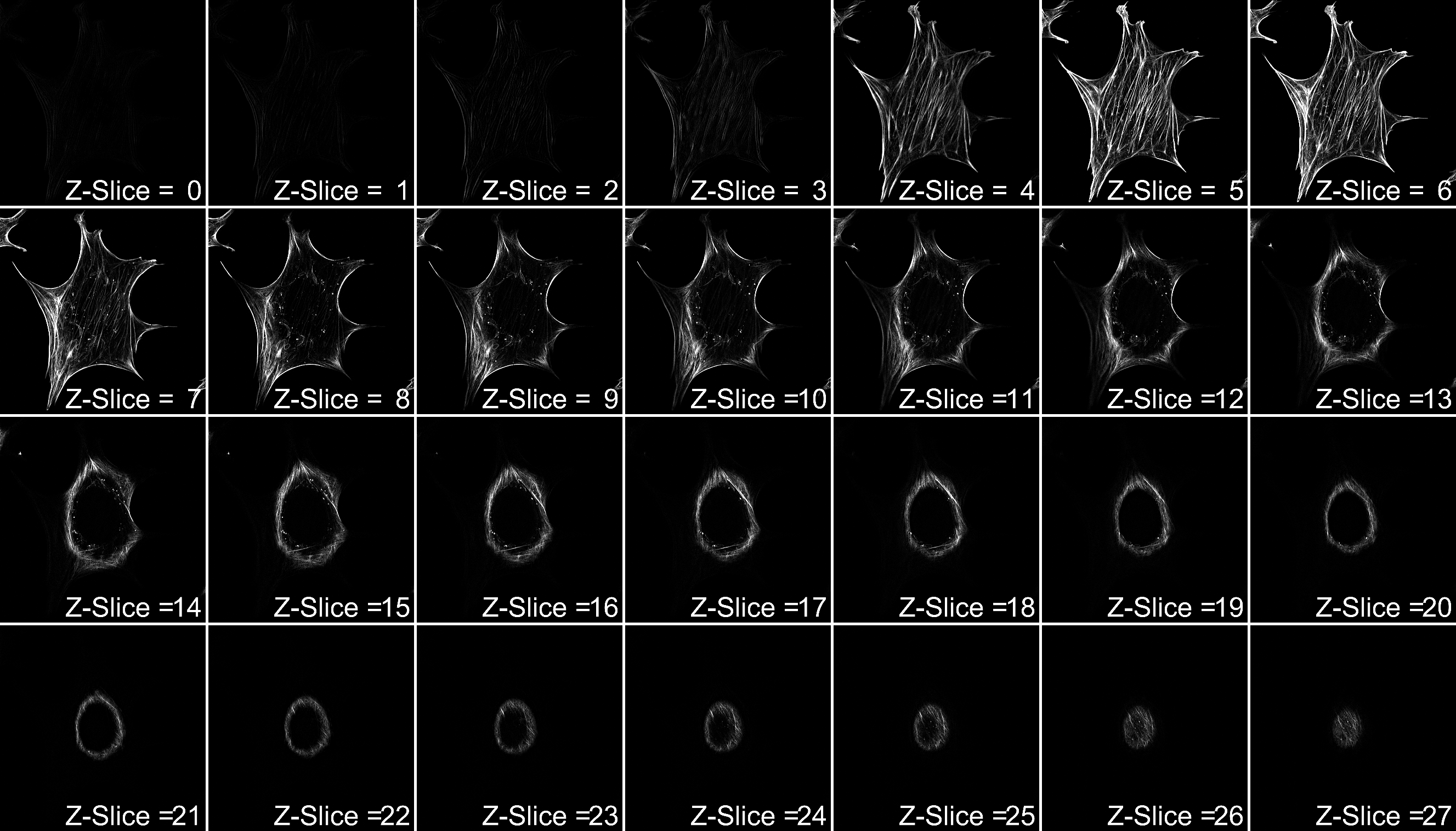
Examples of optical sections of a single cell. Each Z-slice represents 200nm increments.
Source: Nicholas Condon
Colour-coding Depth is also an option for displaying confocal data. Here each slice is colour-coded based upon depth as displayed below.
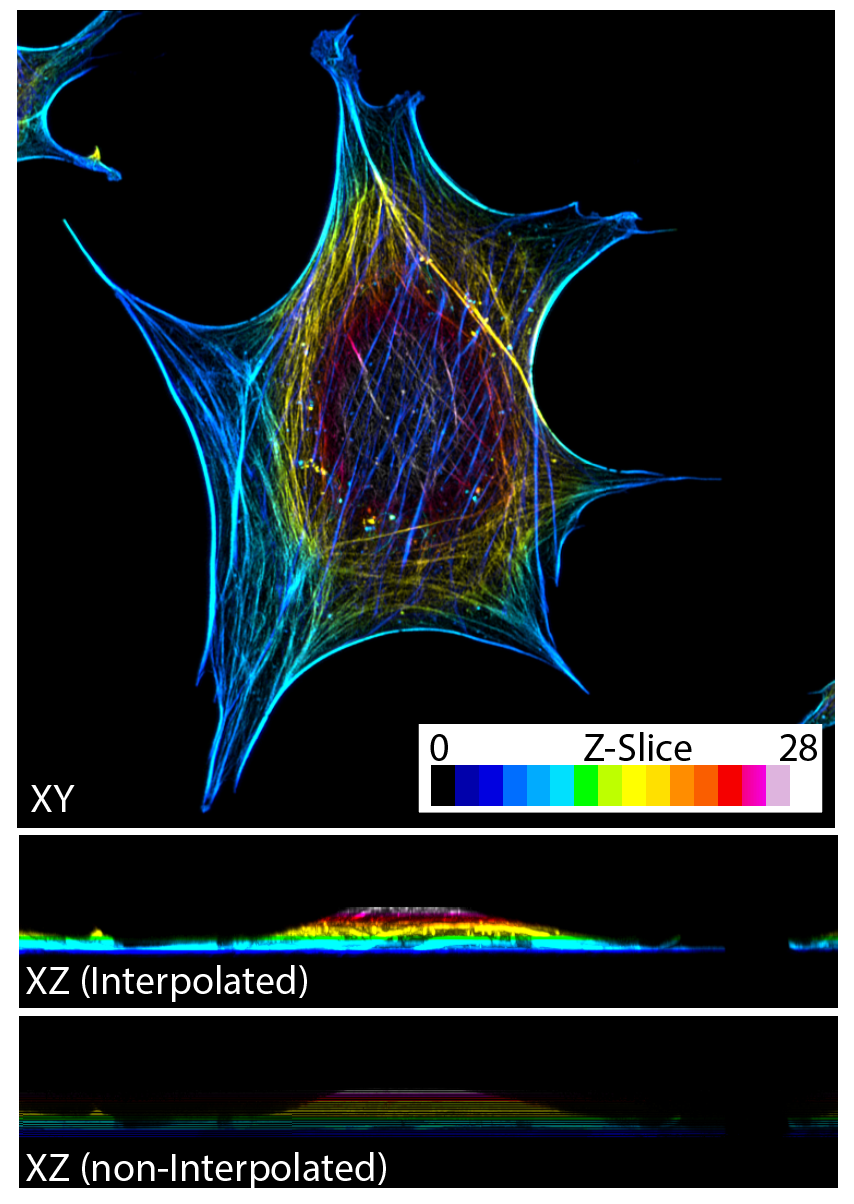
Example of 3D Confocal acquisition represented in XY as a Z-projection with each Z-slice colour coded for depth. XZ examples are presented both interpolated and non-interpolated to show colours labelled by depth.
Laser Scanning Confocal
IMB Systems
- Confocal 1 (Leica SP8 Confocal)
- Confocal 2 (Zeiss 880 LSM Confocal)
- Confocal 3 (Zeiss 710 LSM Confocal)
- Confocal 4 (Zeiss 710 LSM Confocal)
- Confocal 5 (Zeiss 710 LSM Confocal)
Focused beams of laser light are scanned across the sample (Laser Scanning Microscopy, LSM) and light only from the desired focal plane is allowed to enter the detector, filtered by the pinhole. In order to scan the beam back and forth, two mirrors are used; one for left and right (x), and one for up and down (y). Depending on the scan-head, these may be galvanometer mirrors or resonant scanning for higher speed acquisition. In some methods of imaging, the beam can be 'parked' in one position (FCS) or scanned very quickly across a single line for calcium imaging.
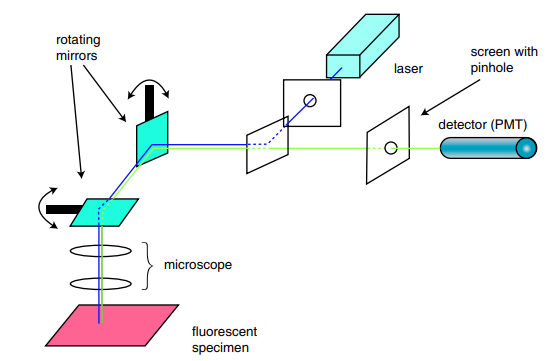
Simplified schematic of a LSM confocal. Note the two rotating mirrors (teal prisms) which direct the point scan, left and right, up and down in order to create the entire image.
Source: DOI: 10.1081/E-EBBE-120024153
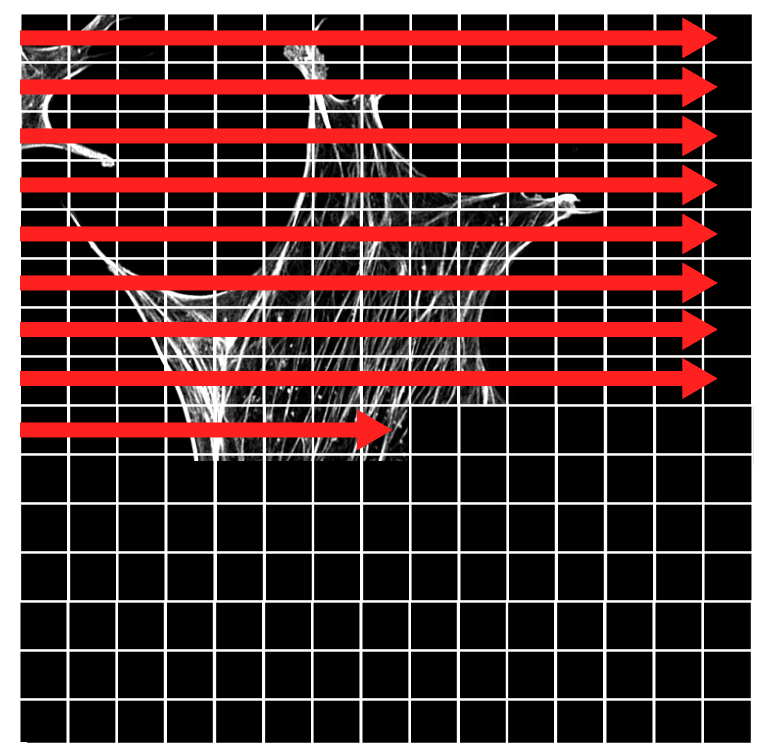
Example of the image rasterization (as it builds up in XY). Note in this example each 'pixel' represented by the grid is a lot larger than in the actual image.
Singe Point Detectors
Because confocals are mostly single point scanners that move very quickly across the field of view, detectors need to be very sensitive as they are only exposed to emission light for microseconds. These detectors are only 1x1 pixel and are often PMT (photo-multiplier tubes). New advances have also occurred to increase sensitivity either as GaAsP (Gallium Arsenide Phosphide) PMTs or as APDs (Avalanche photodiodes). Leica have also produced a HyD detector (hybrid detector, a combination of APD and PMT).
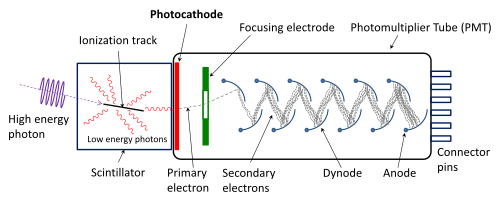
Example schematic of a photon multiplier tube.
Source: Wikipedia
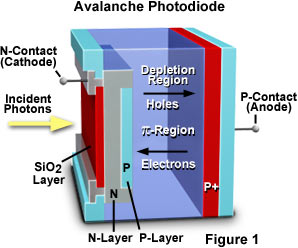
Confocal systems can have different combinations of detectors (ie 2 PMTs and 2 HyDs, or 32 PMTs attached together) for different imaging modalities. There are too many combinations to list here but the most common are spectral detectors (Zeiss 32-channel PMT array), AiryScan Detectors (Zeiss 32-GaAsP spatial detector) or tPMTs (transmitted light PMTs for brightfield).
Note that the same wavelength laser on a variety of different microscopes may have vastly different output powers, so setting the laser on two systems to 2% is not a valid comparison of image intensity even if they are from the same company and you have the detectors set to the same Gain value.
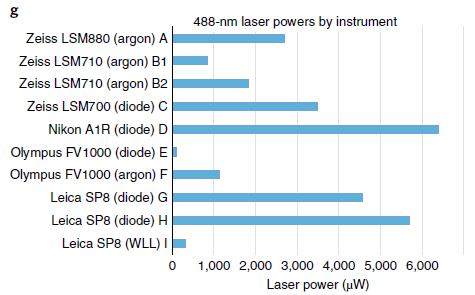
Source Tutorial- guidance for quantitative confocal microscopy.pdf
Spinning Disc Confocal
IMB Systems
- Zeiss Spinning Disc Confocal
- Andor Dragonfly 500 Spinning Disc Confocal
To overcome the issue of speed, spinning disc confocals consist of a high-speed spinning disc with lots of pinholes punched into it which illuminate an entire image (in XY) and create the confocal image onto a camera based detector.
The addition of the microlens array disc means that light is focused down through the pinholes increasing light efficiency. This coupled with sensitive cameras (emCCD or modern sCMOS) enough light is captured when using sufficiently low laser power. One drawback to the spinning disk is that there are multiple pinholes, which means there is a limitation to how much out-of-focus light can be rejected, meaning very thick samples may struggle with a spinning disc (Note: the Andor Dragonfly overcomes this by have two different discs with different sized pinholes).
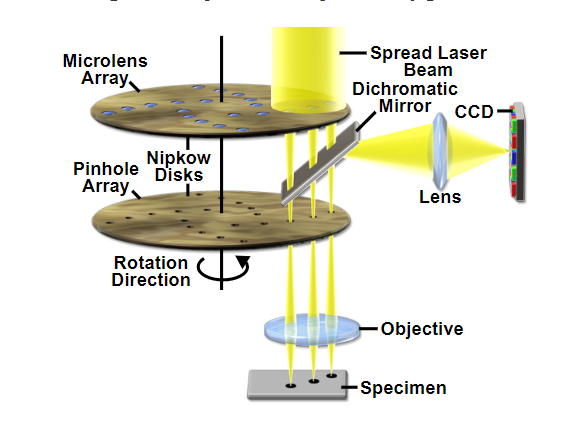
Example schematic of a Yokagowa spinning disc confocal with twin discs (one for pinhole and one for microlens).
Source: MicroscopyU
Microscopy: Optical Sectioning and Confocal Microscopy (Kurt Thorn)
2-Photon Microscopy
IMB System
- Confocal 4 (Zeiss LSM 710 NLO)
Two-Photon microscopy is a method that allows for imaging of very thick samples usually up to 1mm, which is not possible with normal single photon microscopy. For this method, a near infra-red laser is used to minimise light scatter in thick tissue. Because of multiphoton absorption there is very little to no background light, which also improves imaging depth penetration.
Microscopy: Two Photon Microscopy (Kurt Thorn)
Two photon microscopy
