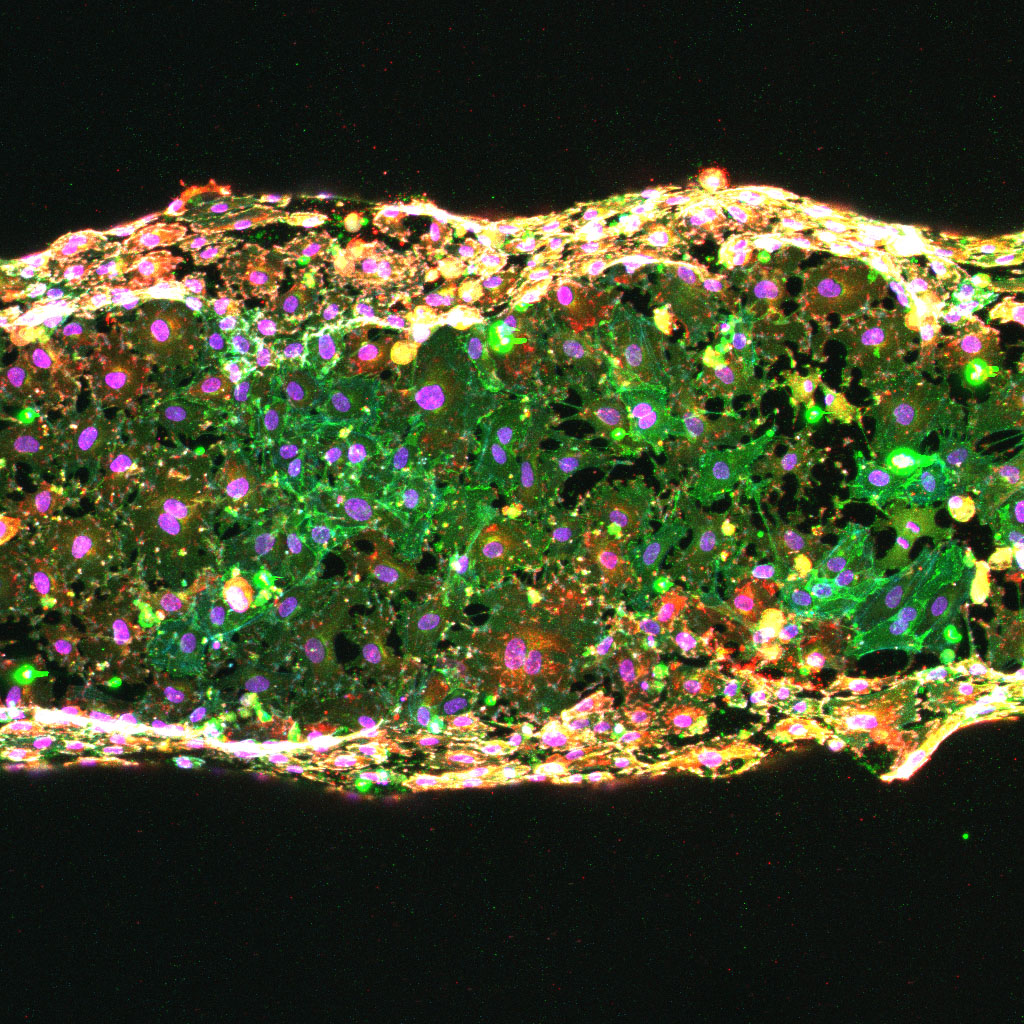
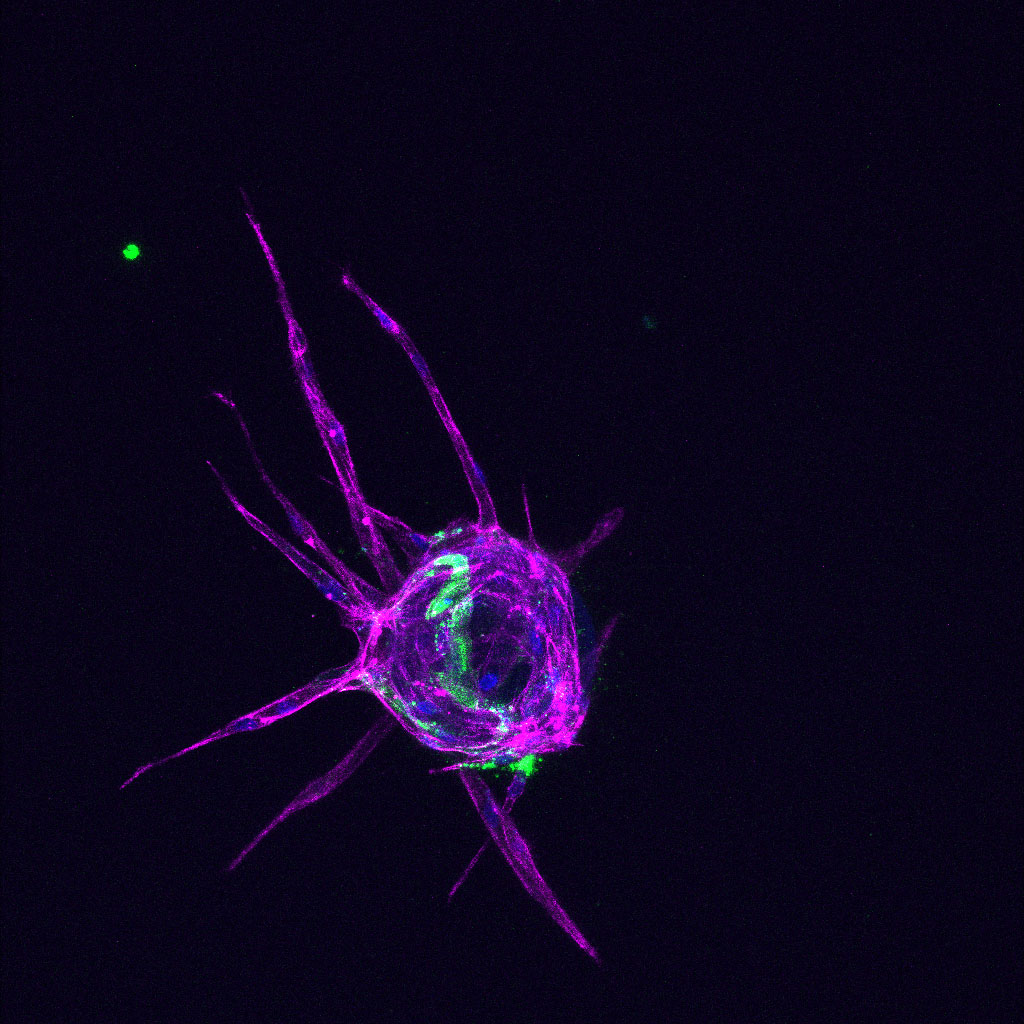
How does the mechanical environment surrounding vessels influence disease progression?
As a result of their function, vessels are subjected to distinct mechanical stresses which confer physical forces on the endothelium, such as fluid flow, stretch and stiffness
Objective/mission (the vision)
In a range of diseases, it is well appreciated that the environment surrounding blood vessels is stiffer than that of healthy tissue. This increased stiffness has been shown to play a central role in promoting a characteristically inflamed and leaky vasculature, driving disease progression. Despite the fact that stiffness is known to induce vessel dysfunction, the signals activated by mechanical forces in disease remain largely unknown.
Research approach (the initiative)
We have identified a central transmitter of signals from the stiff surroundings in pathological environments to induce vascular dysfunction. This vascular dysfunction may include excessive vessel growth and/or breakdown of the vessel barrier. We will use pre-clinical models of disease and 3D bioengineering approaches to identify novel targets that control vascular growth and integrity in stiffness-related diseases.
Impacts and applications
Identifying and targeting abnormal vessel behaviour in response to altered mechanical environments could provide novel approaches to prevent vessel dysfunction in a range of stiffness related diseases, such as cancer and atherosclerosis.
Partners/collaborators
- Professor Paul Timpson, The Garvan Institute of Medical Research, Sydney, Australia
- Dr Maike Frye, University Medical Center, Hamburg, Germany
- Dr Anne Lagendijk, IMB/UQ