The key to discovering new antibiotics and other drugs may lie in the inter-kingdom chemical warfare between fungi and bacteria, according to recent research from the University of Queensland (UQ).
Professor Rob Capon’s laboratory at the Institute for Molecular Bioscience at UQ has discovered a system of chemical warfare between microbes in the sand of Heron Island in Queensland, Australia, which has opened up a new avenue of drug discovery.
Professor Capon says the crux of the research is understanding the process of chemical warfare between bacteria and fungi, to learn how the silent genes coding for microbial ‘molecular weapons’ are activated.
“We discovered that a small defensive molecule released by a fungus acts as a double agent, both attacking the bacteria on behalf of the fungus, while secretly helping the bacteria produce nitric oxide – NO gas,” Professor Capon said.
“The nitric oxide acts as the key to turn on the bacteria's own ‘silent’ genetic molecular defences that go on to successfully defend against the fungal attack,” he said.
These ‘silent’ genes make up around 80% of the potential microbe-fighting molecules in nature and, to conserve energy, these genes aren’t activated unless a microbe is threatened by another known microbe.
“The reason we’ve missed these genes in the past is that we’ve been growing our microbes in monoculture – there’s no threats, there’s lots of food and no competition, so a bacterium won’t turn on its armoury because it doesn’t feel threatened,” Professor Capon said.
Vintage blood pressure drug activates silent genes
Professor Capon and his team determined that the role of nictric oxide in activating chemical defences in bacteria can be reproduced in the lab by simply adding it directly to the culture, bypassing the role of the fungi.
They determined that the vintage blood pressure drug, sodium nitroprusside (SNP), which releases nitric oxide as it breaks down, could be used as a cheap, alternate method to deliver nitric oxide gas directly to the bacteria. This approach was equally effective at stimulating the bacteria’s chemical defences.
“To explore whether SNP could be applied more generally, we took 10 commercially available bacteria – strains whose chemistry had been extensively studied and documented over many decades – and found 50 per cent were stimulated by nitric oxide to produce new chemistry,” Capon said.
“The significance of this going forward is that we can expose pretty much any bacterial strain to nitric oxide, and have an even chance of discovering new (secret) defensive chemistry,” he said.
“This simplicity of this approach heralds a renaissance for the discovery of new antibiotics, and other drugs. For example, rather than left sidelined as an apparent spent force, the huge microbe libraries assembled last century by the pharma industry in their search for new antibiotics can now be brought back in play. Each strain in these libraries can be stimulated by nitric oxide gas to unlock an extraordinary genetic/molecular potential.”
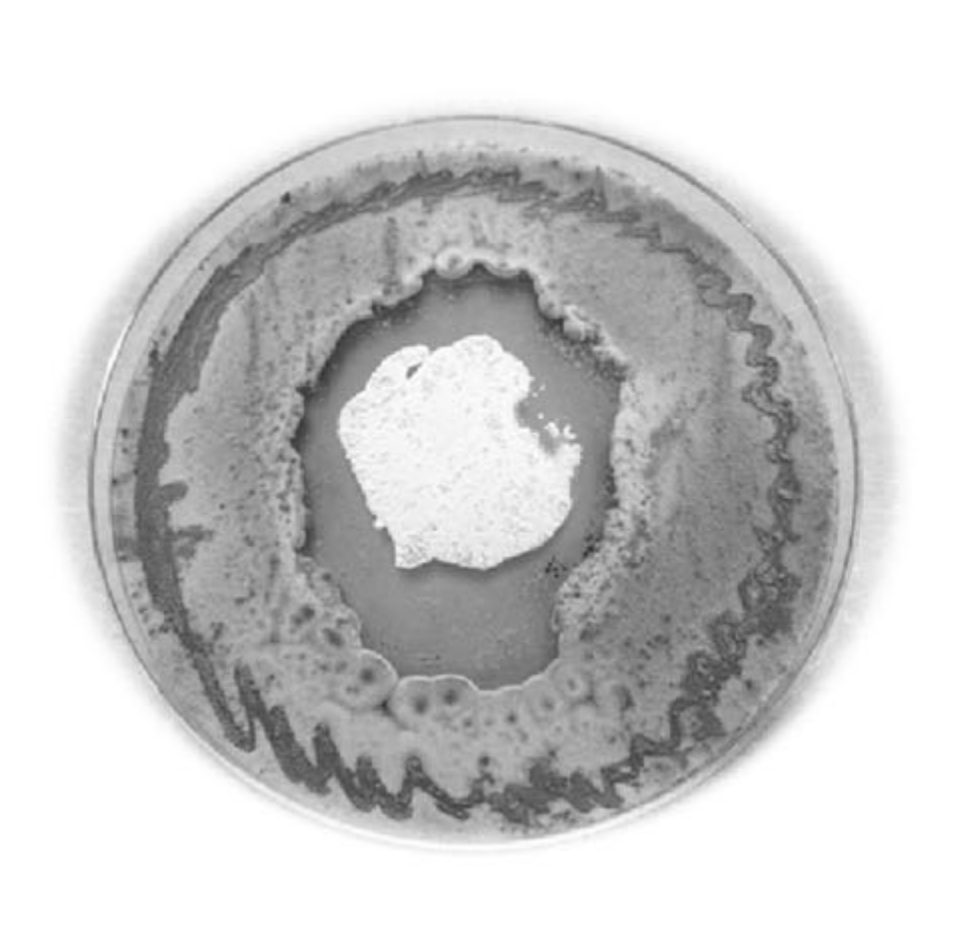
This is a mixed culture of a fungus (the tube-like structure) and a bacteria (the surrounding cloud), with a reagent that glows red in the presence of nitric oxide gas. In the image, the fungus has produced a defensive antibacterial, to which the bacteria responds with nitric oxide (hence the red glow), stimulating the release of a powerful anti-fungal that defeats the fungus. IMB’s Prof Rob Capon is using this new understanding of nitric oxide in microbial chemical warfare to discover new antibiotics. Credit: Dr Nicholas Condon.
He compares the ‘golden years’ of antibiotic discovery in the 1950s and 60s to a gold rush.
“Just as the first to explore a new gold field became rich on the easy surface pickings, so did the first to explore microbes for new antibiotics.”
But ultimately the pharma industry experienced a reduced return on investment due to constant rediscovery of the same compounds over and over again.
“When the surface nuggets are gone – or the easy chemistry, in this case – many move on, as the pharma industry largely did in late last century.
Nitric oxide provides potential new drug discovery process
Armed with a newfound understanding of the drug-discovery potential of nitric oxide, Professor Capon predicts that the time is right to return to the microbial "gold field".
“Using innovative approaches such as nitric oxide, we can mine the mother-load of chemistry hidden away within microbial genomes,” he said.
“Nitric oxide holds great promise for discovering next generation of antibiotics capable of treating infections increasingly resistant to all modern drugs."
The study was published in The ISME Journal (a publication of journal Nature), and was funded by the University of Queensland, Institute for Molecular Bioscience and Australian Institute for Bioengineering and Nanotechnology.