Palpant - clinical and industry partners
Infensa Bioscience (Australia) – Developing novel therapeutics for ischemic heart disease
Stroke and heart attack have two important things in common: (1) they both cause ischemic injury of the heart or brain due to lack of blood supply to the affected tissue, and (2) there are no drugs available to treat the ensuing tissue damage. Infensa Bioscience is developing drugs that directly target the underlying tissue damage and which in preclinical experiments have been shown to massively reduce tissue injury whether they are delivered before or after the re-establishment of blood flow to the brain or heart. These drugs are designed to decrease the mortality associated with these diseases, greatly improve functional outcomes and quality-of-life for survivors, and drastically reduce the burden of these diseases on healthcare systems worldwide. A/Prof Palpant is scientific co-founder and Scientific Advisory Board member.
Relevant Grants
Development of drugs to prevent ischemic injuries of the heart and brain. Medical Research Future Fund Cardiovascular Mission. APP2007625. 2021-2023.
ASIC1a, a new therapeutic drug target for cardiac ischemia. NHMRC Ideas Grant. APP2002857. 2021-2024.
Development of a first-in-class therapeutic for protecting the ischemic heart. NHMRC Development Grant APP2000178. 2021-2023.
Relevant Publications
Redd MA, Scheuer SE, Saez NJ, Yoshikawa Y, Chiu HS, Gao L, Hicks M, Villanueva JE, Joshi Y, Chow CY, Cuellar-Partida G, Peart JN, See Hoe LE, Chen X, Sun Y, Suen JY, Hatch RJ, Rollo B, Alzubaidi MAH, Maljevic S, Quaife-Ryan GA, Hudson JE, Porrello ER, White MY, Cordwell SJ, Fraser JF, Petrou S, Reichelt ME, Thomas WG, King GF*, Macdonald PS*, Palpant NJ*. Therapeutic inhibition of acid sensing ion channel 1a recovers heart function after ischemia-reperfusion injury. Circulation. 2021;144:947–960
HAYA Therapeutics (Switzerland) –
Identifying novel non-coding RNA elements controlling organ fibrosis.
HAYA Therapeutics is a precision therapeutics company that discovers and develops innovative tissue- and cell-selective genomic medicines for fibrotic diseases and other serious health conditions associated with aging, including cancer. The Palpant laboratory is embedded in the company’s discovery engine focusing on identifying long non-coding RNAs (lncRNAs) within the “dark matter” of the human genome to identify key tissue and cell-specific drivers of fibrosis and other disease processes. The long term goal is to develop novel targets and drug candidates with the potential for greater efficacy and safety than existing treatments. HAYA’s lead therapeutic candidate is an antisense molecule targeting Wisper, a cardiac-enriched master driver of fibrosis, which has shown in preclinical testing the ability to halt and potentially reverse the fibrotic processes underlying heart failure.
Relevant Grants
Assessing the role of noncoding RNAs in fibroblast remodeling after cardiac injury. Grant # 31003A_182322. Swiss National Foundation.
Commercial contract with HAYA Therapeutics, Switzerland. Uniquest AG-024694. 2020-2022
Relevant Publications
Shim WJ, Sinniah E, Xu J, Vitrinel B, Alexanian M, Andreoletti G, Shen S, Sun Y, Balderson B, Boix C, Peng G, Jing N, Wang Y, Kellis M, Tam P, Smith A, Piper M, Christiaen L, Nguyen Q, Boden M**, Palpant NJ**. Conserved epigenetic regulatory logic infers genes governing cell identity. Cell Systems. 11, 625-639 e613 (2020).
Merck (Germany) –
Multiplexed single cell RNA-sequencing to accelerate discovery into genetic mechanisms of cell differentiation
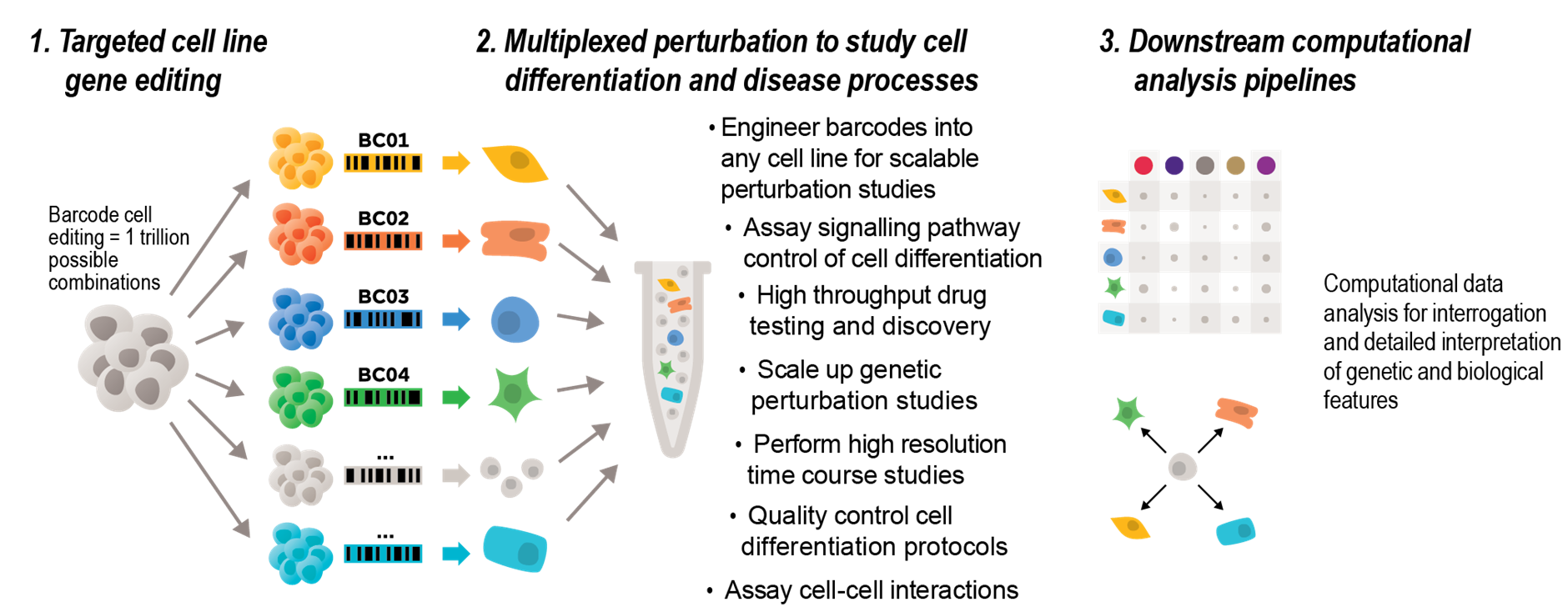
The Palpant laboratory is working with Merck to commercialise a method for barcoding human cells with proprietary barcodes that enables scalable multiplexed single cell RNA-seq. The platform provides unique capabilities for discovery exceeding design limits of existing commercially available barcoding methods. It enables 100 trillion possible unique barcode options to customise complexity and scale of multiplexing, has no sample handling requirements between cell isolation and single cell capture pipelines, involves simple workflow for amplifying barcode library to enhance data quality, and is compatible with any computational demultiplexing method.
The approach expands experimental design options beyond limitations of existing external barcoding. It provides the ability to scale barcoding of any human cell type to maximise data output with current best-practice capture efficiencies, multiplexed analysis of gene, drug, signalling, or other perturbations at scale, parameter testing protocol development such as disease modelling or cell differentiation assays, analysis of high-resolution time-course assays, and dissecting cell-cell interactions by cell mixing assays such as organoid models.
Relevant Grants
Merck Research Grant. 2021-2022
ConcR (UK) – Machine learning methods to improve diagnostic prediction
We are developing methods for statistical inference between multiple imaging and cellular data for predictive computational models. Our goal is identify complex biomarkers, specifically joint imaging-molecular markers, and causal inference methods to identify mechanism of actions to predict therapeutic effects across biological models that can be applied to preclinical in-vitro screening data and retrospective clinical data.
Relevant Publications
Shim WJ, Sinniah E, Xu J, Vitrinel B, Alexanian M, Andreoletti G, Shen S, Sun Y, Balderson B, Boix C, Peng G, Jing N, Wang Y, Kellis M, Tam P, Smith A, Piper M, Christiaen L, Nguyen Q, Boden M**, Palpant NJ**. Conserved epigenetic regulatory logic infers genes governing cell identity. Cell Systems. 11, 625-639 e613 (2020).
Sana Biotechnology (USA) - Advancing stem cells toward clinical testing
Work by Dr Palpant on iPSC genome engineering and differentiation protocols resulted in a licensed patent (US Patent 10,612,002; 2020) on derivation of hPSCs-endothelial cells. This patent and seminal studies on regenerating the mammalian heart with iPSC-derived heart muscle (Nature) formed the basis for Sana Biotechnology (USA; USD $700M series A VC investment in 2019). The Palpant group is working with Sana CSO Professor Charles Murry to advance methods that control cell differentiation and improve engraftment of cells during transplantation.
Relevant Grants
Induced pluripotent stem cell derived cardiomyocytes: a new therapy for "no-option" end stage heart failure. Medical Research Future Fund Stem Cell Therapies Mission. APP2007625. 2021-2025.
Global Strategy and Partnerships Award, The University of Queensland, 2017, 2019
Relevant Publications
Friedman CE, Nguyen Q, Lukowski SW, Chiu HS, Helfer A, Miklas J, Suo SS, Han JDJ, Osteil P, Peng G, Jing N, Baillie GJ, Senabouth A, Christ AN, Bruxner TJ, Murry CE, Wong ES, Ding J, Wang Y, Hudson J, Ruohola-Baker H, Bar-Joseph Z, Tam PPL, Powell JE**, and Palpant NJ**. Single-Cell Transcriptomic Analysis of Cardiac Differentiation from Human PSCs Reveals HOPX-Dependent Cardiomyocyte Maturation. Cell Stem Cell. 2018 Oct 4;23(4):586-598.e8.
Palpant NJ*, Pabon L, Friedman CE, Roberts M, Hadland B, Zaunbrecher RJ, Bernstein I, Zheng Y, Murry CE. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nature Protocols, 2017 Jan;12(1):15-31.
Chong JJ, Yang X, Don CW, Minami E, Liu Y, Weyers JJ, Mahoney WM, Van Biber B, Palpant NJ, Gantz J, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem H, Laflamme MA, Murry CE. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate Non-Human Primate Hearts. Nature. 2014 Apr 30.
Shiba Y, Fernandes S, Zhu W, Kim J, Palpant NJ, Gantz J, Moyes KW, Muskheli V, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Murry CE, Laflamme MA. Human ESC-Derived Cardiomyocytes Electrically Integrate and Suppress Arrhythmias in a Guinea Pig Infarct Model. Nature. 2012 Sep 13;489(7415):322-5.
