“What doesn’t kill me makes me stronger”, originally coined by Friedrich Nietzsche in 1888, is a perfect description of how bacteria develop antibiotic resistance.
Contrary to a common belief, antibiotic resistance is not about your body becoming resistant to antibiotics.
Resistance arises when bacteria are exposed to levels of antibiotics that don’t immediately kill them. They develop defences that prevent the same antibiotic from harming them in the future, even at higher doses.
How bacteria adapt
The ability for bacteria to adapt lies in part with their astonishing rate of reproduction. Some species, such as Escherichia coli, can replicate as quickly as every 20 minutes, depending on the environment. One bacterium can become more than 68 billion bacteria in 12 hours.
However, bacteria don’t faithfully reproduce their genetic code, and mutations can slip in every generation.
While most changes are bad, sometimes they can help the bacteria grow in the presence of an antibiotic. This “new and improved” population quickly takes over.
Additional mutations enable survival at even higher antibiotic concentrations.
This evolution of resistance can be seen by growing bacteria on a large agar plate (a nutrient support that bacteria like to grow on) with zones of increasing antibiotic levels.
Growth is halted when they first encounter the next zone, but once they have developed resistance they quickly expand until they reach the next region with more antibiotic.
Bacteria in your body can easily develop resistance in a similar manner during the typical seven- to ten-day course of antibiotic treatment.
They also exchange genetic material
The other key mechanism enabling bacterial resistance is the exchange of genetic information between bacteria.
In addition to the main chunk of DNA that encodes the bacterial genome, bacteria can host circular DNA snippets called plasmids. These plasmids are readily exchanged between bacteria, including different species.
Plasmid exchange usually occurs by direct physical contact between bacteria. Bacteria are promiscuous, so this can happen a lot! Once inside a bacteria, plasmids can be passed down to the next generation.
Unfortunately, plasmids are particularly good at encoding multiple resistance genes.
Four ways bacteria resist
Bacteria develop resistance to antibiotic treatment using four main methods:
1) Keep the antibiotic out. Bacteria are good at keeping unwanted molecules from getting inside.
Gram-positive bacteria like Staphylococcus aureus have a thick cell wall enclosing a lipid membrane. Gram-negative bacteria, such as E. coli, are more difficult to kill as they have an additional outer membrane that acts as an extra barrier.
Bacteria are able to bring in the things they need to survive through these cell surfaces. Antibiotics can hijack these entry routes, but bacteria can modify the cell wall, cell membrane and entry proteins to block antibiotic penetration.
For example, bacteria increase the thickness of the cell wall to resist antibiotics like vancomycin.
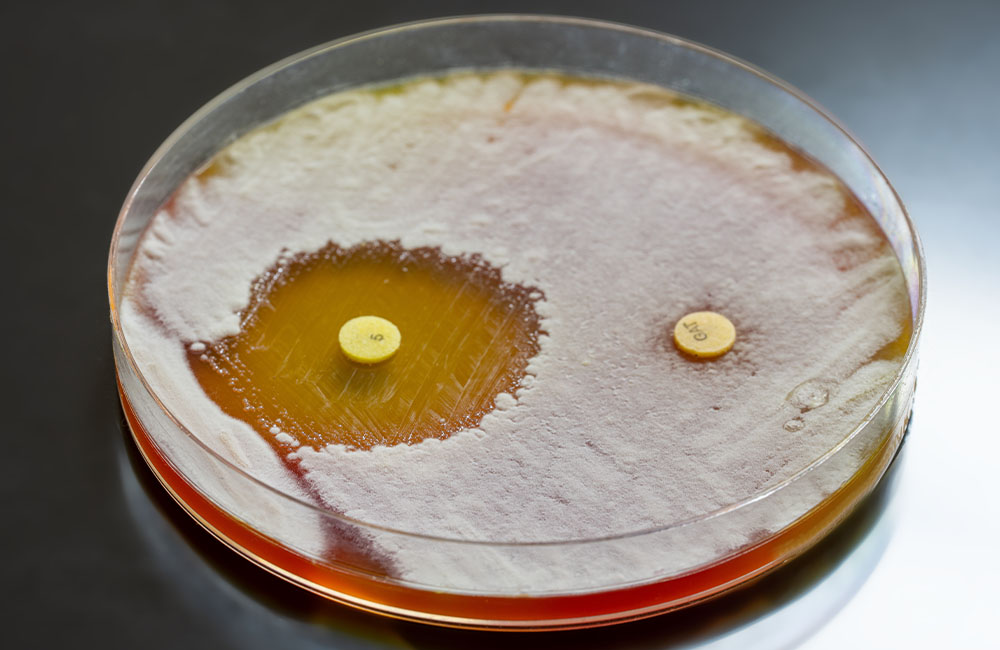
2. Expel the antibiotic if it gets in. Bacteria have machinery known as efflux pumps, which regurgitate unwanted molecules from within the bacteria.
Bacteria can alter the pump so it is more effective at removing the antibiotic, or they can simply make more pumps.
Resistance to macrolide antibiotics like erythromycin often involves the production of more efflux pumps.
3) Alter the antibiotic target. Antibiotics, like most other drugs, generally work by blocking the function of important enzymes within the bacteria. They specifically bind to the target like a key in a lock.
If bacteria alter the target shape by changing the DNA/protein sequence, the antibiotic (key) can no longer bind to its target (lock).
Resistance to a class of antibiotics known as fluoroquinolones (which includes ciprofloxacin) often occurs due to mutations of the enzyme targets.
4) Destroy or modify the antibiotic. Bacteria developed resistance to the original antibiotic, penicillin, by producing a protein that breaks apart the penicillin warhead.
These enzymes have evolved to keep pace with even the most recent new and improved penicillin-like antibiotics.
In response, drug developers have created molecules that specifically stop the enzyme from working, and dose these in combination with the antibiotic.
Another example of antibiotic modification is shown by resistance to a class of antibiotics called aminoglycosides. In this case, different types of enzymes chemically modify the structure of the aminoglycoside, such as the antibiotic tobramycin. Now, the key has been filed so that it no longer fits the lock.

Bacteria vs antibiotics
While bacteria have developed mechanisms to resist antibiotics, these adaptations can come at a “fitness” cost. Bacteria may grow more slowly, or can be killed more easily by another antibiotic.
This has led to the concept of “collateral sensitivity” to prevent or overcome resistance when treating patients, by using pairs of antibiotics. Resistance to the first antibiotic increases susceptibility to the second, and vice versa.
In some cases, the “fitness costs” (energy and materials expended to maintain resistance) mean that resistance genes can be present, but they are not activated until exposed to an antibiotic. This makes it difficult to predict bacterial resistance by just looking at their genetic makeup.
Bacteria may get “stronger,” but they are not yet invincible. We need to take action before antibiotic resistance returns us to a pre-antibiotic era.
 Written by Professor Mark Blaskovich.
Written by Professor Mark Blaskovich.
This article is republished from The Conversation under a Creative Commons license. Read the original article.