IMB's Director, Professor Ian Henderson, Professor of Microbiology and IMB researcher Dr Larisa Labzin, discuss COVID-19: immunity, vaccine development, epidemiology and what we do and don’t know.
-
What is herd immunity and can we use it to defeat the virus?
-
Should healthy people wear a mask?
-
What does "flattening the curve" mean and why is it important?
-
If you survive COVID-19 after an infection, are you protected with immunity?
-
Do you need to experience lower respiratory symptoms like bronchitis to mount a protective antibody response needed for immunity?
-
Why are kids under-represented among more severe COVID-19 cases?
-
Why Australia hasn't moved to general school closures?
-
Is Australia heading for the same situation as Italy?
-
What is the current situation like for the Australian healthcare system and will it hold up?
-
What works to contain the spread of COVID-19?
-
What happens when social distancing restrictions are lifted?
-
Why does it take such a long time to make a vaccine?
-
How would a coronavirus vaccine work?
-
Are there examples of other coronavirus vaccines we can look to?
-
What lessons can we learn from COVID-19?
What is herd immunity and can we use it to defeat the virus?
Professor Ian Henderson:
Herd immunity protects those who cannot mount an immune response or those in whom immunity will not work, usually in the context of a vaccinated population, rather than after natural infection. For some infections, 95% of the population needs to have been vaccinated to provide herd immunity.
In the case of COVID-19 we have yet to establish what proportion of the population needs to have been infected, and recovered, to cease transmission of the virus amongst the population. However, worst case scenario estimates suggest that over 80% of the population would need to be naturally infected and recovered to cease transmission between people.
The key point about herd immunity is that the protected population provide a shield to reduce the transmission of the virus to the vulnerable and those who cannot clear the infection. We experience this in our day to day lives, as it helps protect us from diseases like measles. '
Using a conservative case fatality rate of 1%, this could result in approximately 500,000 deaths in the UK for example; but this could be reduced if the at-risk populations, the elderly and those with comorbidities, were to isolate themselves throughout the pandemic.
Dr Larisa Labzin:
As much as viruses have a reason for exisiting, the virus that causes COVID-19 (SARS-CoV-2) is just trying to replicate as much as possible. To do that most efficiently it needs to find somewhere to replicate (i.e. a person).
If it kills the person (as in severe cases) it can’t replicate. If the immune system kicks in, it stops the virus replicating. So the virus is trying to find new people to replicate in that are a) living and b) haven’t got immunity yet. If you consider a total population, as the number of people who have been infected increases, the number of ‘fresh targets’ decreases. As the number of recovered and immune people increases, the chances for the virus to replicate also decrease.
If you reach a certain percentage of people in the population being immune, the virus can’t find new people to replicate in, and even if there are non-immune people, the virus is physically separated from them (because an infected person and the non-immune person will statistically never meet).
This will theoretically happen naturally in the pandemic, but it will kill a lot of people along the way. With vaccines, we can create immunity without needing to be infected, so we can protect people. With the coronavirus because we don’t yet have a vaccine, herd immunity will only happen if a lot of people get sick and recover. This will put very vulnerable (especially older people) at risk of dying. Social distancing will help SLOW the virus spread because it means the virus is less able to find new people to replicate in quickly.
Should healthy people wear a mask?

Professor Ian Henderson:
Respirators, which filter particulates from the air, do have demonstrable protective effects for healthcare workers in the context of respiratory disease, such as influenza. Surgical masks also appear to offer some protection. This is important as the potential exposure of healthcare workers to infectious agents is much higher than in the general population.
In contrast, there are several conflicting studies about the effectiveness of face masks in preventing community acquired infections among the general population. Many of the studies conducted so far have been on small numbers of individuals that prevent robust evaluation of the data.
It is important to note that we still do not have robust data on how the coronavirus transmits between people. It may be that the use of masks by non-infected individuals offers limited value in controlling the spread of infection within the community.
In contrast, it is important for infected people to wear masks so that they reduce the amount of virus they release into the environment.
We should be careful as masks may give a false sense of security, they may be worn inappropriately, they can promote touching of the face — all of which give rise to a higher risk of infection.
Further, if the general population depletes supplies of face masks it may have a devastating effect on the availability of masks for those who need them most; our frontline healthcare professionals.
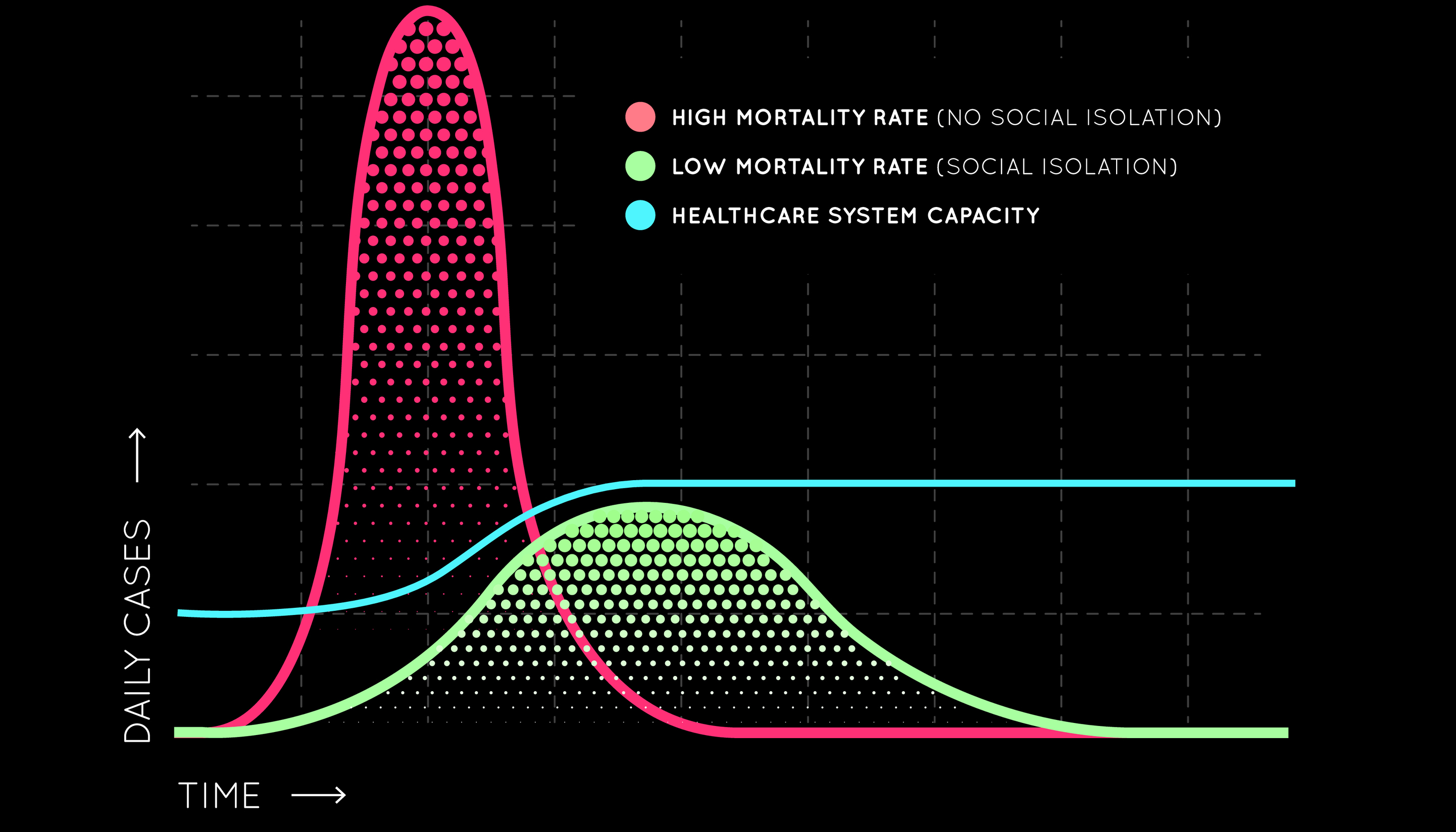
What does "flattening the curve" mean and why is it important?
Professor Ian Henderson:
Hospital services have limited numbers of healthcare workers, such as nurses and doctors, limited numbers of beds, limited numbers of specialist equipment, such as ventilators. By flattening the curve, it allows healthcare workers and the available infrastructure, time to cope with the number of patients. Modelling indicates that without intervention, 50% of cases will occur within 3 weeks of sustained person to person transmission and 95% of cases will occur within 9 weeks.
Dr Larisa Labzin:
"Flattening the curve" means that instead of having a really rapid spread of the virus, where everyone gets really sick all at once, through isolation and quarantine we slow the spread of the virus. Without measures in place we would have a LOT of cases very quickly – which is far more than our health systems can handle. That is what is happening in Italy right now with doctors unable to treat everyone. If we flatten the curve it means that the pandemic will go on for longer but the number of cases at the same time will be less. This means less burden on our healthcare system, meaning better care for the patients who get very sick. It also gives us more time to develop a vaccine.
If you survive COVID-19 after an infection, are you protected with immunity?
Professor Ian Henderson:
The answer is that we do not know yet.
It seems likely that infection will generate protective immunity, at least in the short-term, and in the absence of virus mutation.
There have been rare reported cases of reinfection, where an infected individual seems to have recovered but then tests positive for the virus later, the reasons for this are unclear. However, the general consensus amongst experts is that recovery from infection is likely to result in subsequent protection; the reinfection observation is unlikely to have occurred and probably results from a testing regime that gave a false negative result at the time of recovery.
Dr Larisa Labzin:
Yes we think so.
There is evidence of an antibody response (sero-conversion) and the serum from people who have recovered from SARS-CoV-2 is able to neutralise the virus.
What we don’t know is how long this protection lasts. From SARS (2003 pandemic) and MERS we think that immunity is strong up to a year after initial infection. But we really don’t well enough yet.
We should be able to retrospectively track who has had SARS-CoV-2 by looking for antibodies against the virus in people’s serum. This is what happens when we are tested for having protection against other vriruses. e.g. hepatitis.
A test for SARS-CoV-2 antibodies has been developed and should hopefully be in use soon.
Do you need to experience lower respiratory symptoms like bronchitis to mount a protective antibody response needed for immunity?
Professor Ian Henderson:
It is too early a stage to understand exactly what is happening with the immune response to COVID-19 in infected patients. However, based on experience with other infections, it is unlikely that a lower respiratory infection would be required to mount a protective immune response.
Dr Larisa Labzin:
Good question. I'm pretty sure that you would get a protective response even if it’s just in the upper respiratory tract. The mucosal response generated will also mean that your immune cells in the blood should have seen the virus.
Why are kids under-represented among more severe COVID-19 cases?
Professor Ian Henderson:
One of the most interesting facets of this disease is that kids have been spared the fatal consequences of infection. This should be viewed as one of the few positives associated with the outbreak. The basis for this sparing is not understood, but we do know that children can get infected, can present with fever or remain asymptomatic. In either case, we have a limited knowledge of the degree they can transmit the virus to others.
Dr Larisa Labzin:
We don’t know why kids aren’t getting really sick.
What distinguishes how sick you get is how the immune system responds – and we think in severe cases of COVID-19 it’s the overacting immune system causing part of the damage. This happens with severe cases of flu too.
How the immune system responds depends on what it has seen before – therefore in younger patients it might be less likely to mount a really severe response because it hasn’t seen as many different viruses before. This is both at the level of the humoral response (i.e. we might have antibodies against other circulating coronaviruses that are cross reactive but not protective) but also in our other immune cells, that might respond differently if they’ve seen lots of viruses before.
It might also be that in general children are better able to repair the damage to their lungs caused by the virus (or by the immune system trying to fight the virus). This is because they are undergoing growth and have these processes running pretty efficiently anyway.
We don’t really know why children are more susceptible to some respiratory viruses (e.g. influenza, RSV) but resistant to others (e.g. coronaviruses). It could be a bunch of these possibilities and we won’t know until we are able to study the virus in more detail and the immune response it generates.
Why Australia hasn't moved to general school closures?
Professor Ian Henderson:
Closing schools works for seasonal flu, because children are susceptible to infection, but it does not necessarily work for all outbreaks.
We do not know about coronavirus, which shows a relative sparing of children, in terms of serious disease.
Experience from the 1918 flu pandemic did not indicate significant benefits from closing schools.

Given the longevity of the incubation period for coronavirus it may only have a marginal impact of flattening the curve. If excluded from school for a 13-week period it is likely that the children would mix anyway, and if they are carriers, transmission would be sustained. The flow on impact to the economy and healthcare system could be significant.
The answer is not always “do it now”. Certainly, older citizens and those with underlying comorbidities need to be protected from exposure to the virus, and that protection must last over the peak of cases.
Those most at risk, the elderly and those with comorbidities should consider isolating themselves from potential sources of infection, this includes children.
Is Australia heading for the same situation as Italy?
Professor Ian Henderson:
Australia is very different to Italy.
It is geographically isolated.
It is 26 times larger.
It has a third of the population.
It is difficult to make predictions but given these factors transmission may be slower in Australia, and therefore a flatter peak is possible.
What is the current situation like for the Australian health care system and will it hold up?
Professor Ian Henderson:
This is difficult to comment on as we are research scientists, not healthcare workers. However, Australia has a 1st class, well-developed health care system and can provide a co-ordinated response.
The system is designed to flex in response to crises and there is no indication that the health care system could not cope. However, the portion of the health care system likely to see the greatest pressure will be that with dealing with respiratory care. There will be additional pressures across the system as health care workers contract the disease, resulting in high rates of absenteeism, and as other practitioners are called in to support critical care and units responding to the pandemic.
What works to contain the spread of COVID-19?

Professor Ian Henderson:
Effective handwashing is essential.
The use of soap and proper handwashing for at least 20 seconds is the most effective means of removing virus particles from your hands. In the case of children, supervised hand washing may be appropriate.
Avoid touching the face.
While there is much still to learn about the route of transmission, it is possible that this virus is not only spread through inhalation of viral particles but may enter the body through other soft tissue routes such as the eyes, lips etc. It is also possible that the virus is shed in faeces and so washing hands after visiting the bathroom is crucial.
Physical distancing
Previous experience, such as the 1918 influenza pandemic, have revealed that physical distancing approaches (previously called social distancing) were effective in past pandemics in controlling diseases.
The efforts of the Chinese state in implementing and enforcing physical distancing have been remarkable in supressing the number of cases. They have taken extraordinary steps to protect their populations and to prevent the spread of the virus. These extreme physical distancing measures have worked in the immediate term.
What does physical distancing mean?
It means not attending mass gatherings, maintaining approximately a distance of 2 metres from others when possible, not holding face-to-face meetings, not entering enclosed spaces and avoiding physical contact.
Government interventions, like event closures, play a crucial role, however individual behaviours are even more important.
Healthy people should stay 6 feet away from other people whenever possible. They should reduce unnecessary interactions; work from home where possible, have meetings via the internet, avoid physical contact etc.
Those that are ill should isolate themselves to protect others, this means staying home, not welcoming visitors into your home, and avoiding sharing household items.
It does not mean you cannot enjoy life.
Walking in the countryside or playing golf, are examples of activities that are sufficiently distant from others to allow appropriate physical distancing.
Dr Larisa Labzin:
Stay home and self isolate where possible, especially if you appear sick.
We also don’t know if we are carrying the virus so we could be infecting people accidentally because we don’t know that we are sick.
Wash hands and practice good hygiene as recommended.
What happens when social distancing restrictions are lifted?
Professor Ian Henderson:
The caveat for extreme social distancing, as seen in China, is that as soon as the restrictions are lifted there is the potential for a second wave of infections as people who were naïve to the infection suddenly encounter the virus and transmission occurs again.
This may result in apparent higher mortality rates as those who are susceptible stop self-isolating and rejoin the general population.
Why does it take such a long time to make a vaccine?
Professor Ian Henderson:
Vaccines are the single largest contributor to increased life expectancy in the last century. However, the development of safe and effective vaccines is challenging.
An effective vaccine must boost immunity to the infectious agent and must sustain protection over long periods. The vaccine must protect those groups of people most at risk such as the elderly. In addition, the vaccine must not exacerbate the infection by causing immunopathology on exposure to the infecting agent. Finally, the antigens for the vaccine must be capable of being produced in large quantities.
Unfortunately, it simply takes time to develop the vaccine, to ensure its safety and efficacy, and to produce sufficient quantities.
Arguably, the vaccine we are most familiar with is the seasonal flu vaccine. It is produced in millions of doses every year using a well-established process. Yet, even with the pathways for production, and with safety and efficacy procedures already established, it takes 6 months to produce.
Dr Larisa Labzin:
A vaccine is essentially tricking the immune system into thinking its seen the virus before. This means we have all the protective immune responses without having to get sick in the first place.
First we have to engineer the vaccine, then we have to see that it works as we expect (e.g. it triggers an antibody response), then we have to see if that is protective against the virus in animal models, then we have to test different doses to see if it’s a) safe and b) effective in clinical trials, then we have to make lots of the vaccine very quickly.
This usually takes a long time (up to ten years) but because of great efforts, researchers think they can make a vaccine in 12 to 18 months.
How would a coronavirus vaccine work?
Professor Ian Henderson:
With the emergence of 2019-nCoV, there are now 15 potential vaccine candidates in the pipeline globally using a wide range of technology — mRNA, DNA, nanoparticle, synthetic and modified virus-like particles.
It will take at least six months for most of these potential vaccines to start phase 1 clinical trials; the vaccines funded by Coalition for Epidemic Preparedness Innovations (CEPI), such as the vaccine being developed by The University of Queensland, are likely to reach trials the quickest.
The next challenge will be finding enough production capacity globally to produce these competing vaccines, at a scale that millions or even billions of people can be vaccinated.
Dr Larisa Labzin:
Ideally a vaccine will show the immune system important bits of the virus so that the immune system uses it as a template to make antibodies against the real virus.
Some of the technology involves making nanoparticles that have just one of the virus proteins expressed (so it looks like the virus but isn’t able to replicate).
Alternatively researchers will put little bits of the SARS-CoV-2 into viruses that are really safe (not able to replicate) so that the immune system would make protective antibodies and have a protective T-cell response against the virus.
The Coalition for Epidemic Preparedness Innovations (CEPI) is funding at least 6 different companies/academic groups to use their different technologies to make a coronavirus vaccine.
Are there examples of other coronavirus vaccines we can look to?
Professor Ian Henderson:
The majority of potential SARS (Severe Acute Respiratory Syndrome) or MERS (Middle East respiratory syndrome) vaccines have been investigated in cell or animal models and it is difficult to extrapolate results from these models to know if they would be effective in humans.
Four potential SARS/MERS vaccines have entered Phase I clinical trials to test for safety. All of these formulations were found to be safe and well tolerated. In short-term experiments the vaccine candidates induced appropriate immune responses in the participants; however in the one long term experiment the ability of the immune response to neutralise the virus waned over time.
Due to the genetic relatedness of the viruses, there may be some cross-protection from using a SARS/MERS vaccine while awaiting COVID19 vaccine. However, this would require close monitoring of small groups of vaccinated individuals in areas where there is active transmission of the current virus.
What lessons can we learn from COVID-19?
Professor Ian Henderson:
The emergence of another coronavirus, after SARS-CoV and MERS-CoV suggests these viruses may pose a continuing threat to human beings.
This epidemic will eventually peter out, and as history demonstrates, people's memory of the scale and difficulties posed by the current outbreak will gradually blur. However, we must not forget the lessons learned from this crisis.
The lessons learned now will provide a roadmap for the response to future outbreaks.
Governments must have the strength to implement drastic measures and rapidly mobilize resources to prevent the spread of the virus and to care for the sick.
However, epidemic preparedness and the ability to rapidly produce new vaccines is a critical step in preventing the next pandemic.
Currently, the UK and many Western countries lack a “single source of truth” for disease control, similar to the Centers for Disease Control and Prevention in the USA. The cost of building and sustaining such an entity to deal with crises such as this, is a tiny fraction of the money the government is now spending to keep the economy afloat, and is an even smaller portion of the monetary impact on the economy as a whole.
Government should work to establish a national centre for vaccine development such that we can respond rapidly in the future. This should be done in conjunction with those who have experience in developing vaccines and bringing them successfully to implementation in large populations.


Professor Ian Henderson answered these questions with Professor Adam Cunningham, Professor of Functional Immunity at the University of Birmingham, and Director of the multinational BactiVac Vaccine network.