How does intracellular trafficking guide cell movement and vessel growth?
Blood vessel sprouting is involved in general tissue homeostasis, and deregulation of this critical process results in developmental defects or a wide range of pathological diseases such as cancer and diabetic retinopathy.
Objective/mission (the vision)
This project aims to identify how signalling pathways within cells lining the blood vessel wall (endothelial cells) regulate migration via internalisation and secretion of proteins important for vessel sprouting. By identifying these signals, we can find ways to manipulate them to prevent excessive sprouting in disease.
Research approach (the initiative)
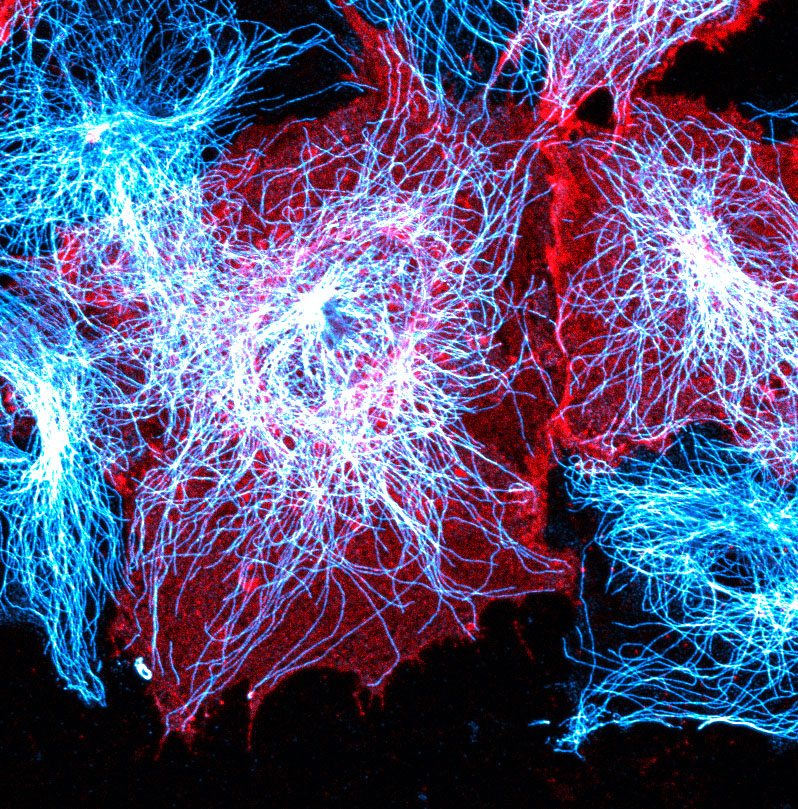
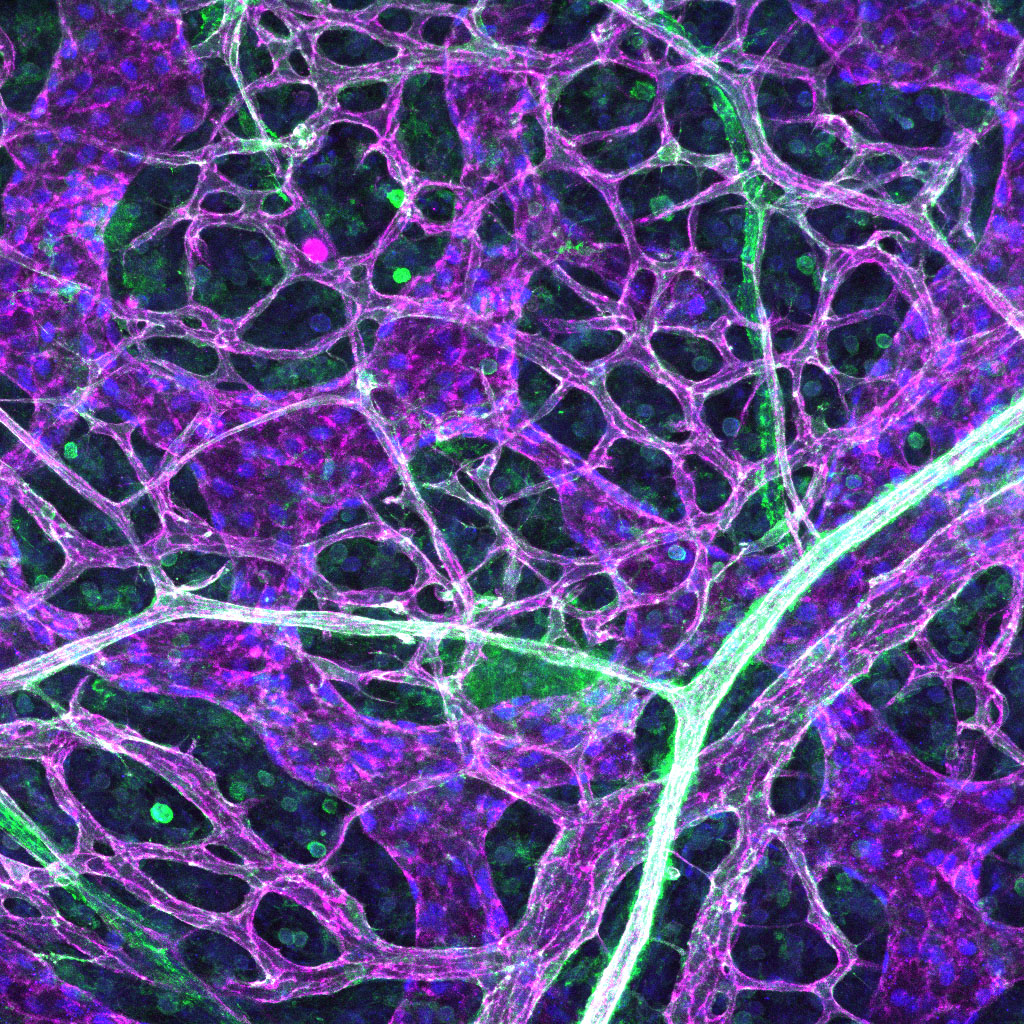
Migration of endothelial cells is established by the cell’s cytoskeleton creating pushing and pulling forces and by cells grabbing on to their surroundings. The cytoskeleton not only regulates cell migration but also affects how proteins are internalised and secreted by cells, which subsequently can change a cells migratory behaviour. Yet how this occurs in blood vessels remains unknown. We have identified signals in endothelial cells, that when activated, lead to altered trafficking of intracellular components through changes in the cytoskeleton. Activation of these pathways leads to highly abnormal and leaky vessels, similar to what is observed in a number of disease states. To further investigate how internalisation and secretion of proteins are altered by the cytoskeleton, we use high resolution and high-speed imaging of cell biological events in real time, and then assess how this can regulate vessel sprouting in 3D models.
Impacts and applications
Uncovering the molecular interactions and trafficking events that cause vascular dysfunction will allow us to identify novel targets to prevent vessel growth in diseases such as cancer and pathological eye disease.
Partners/collaborators
- Dr Samantha Stehbens, IMB/UQ
- Professor Alpha Yap, IMB/UQ