How is blood vessel integrity controlled across specific vessel types?
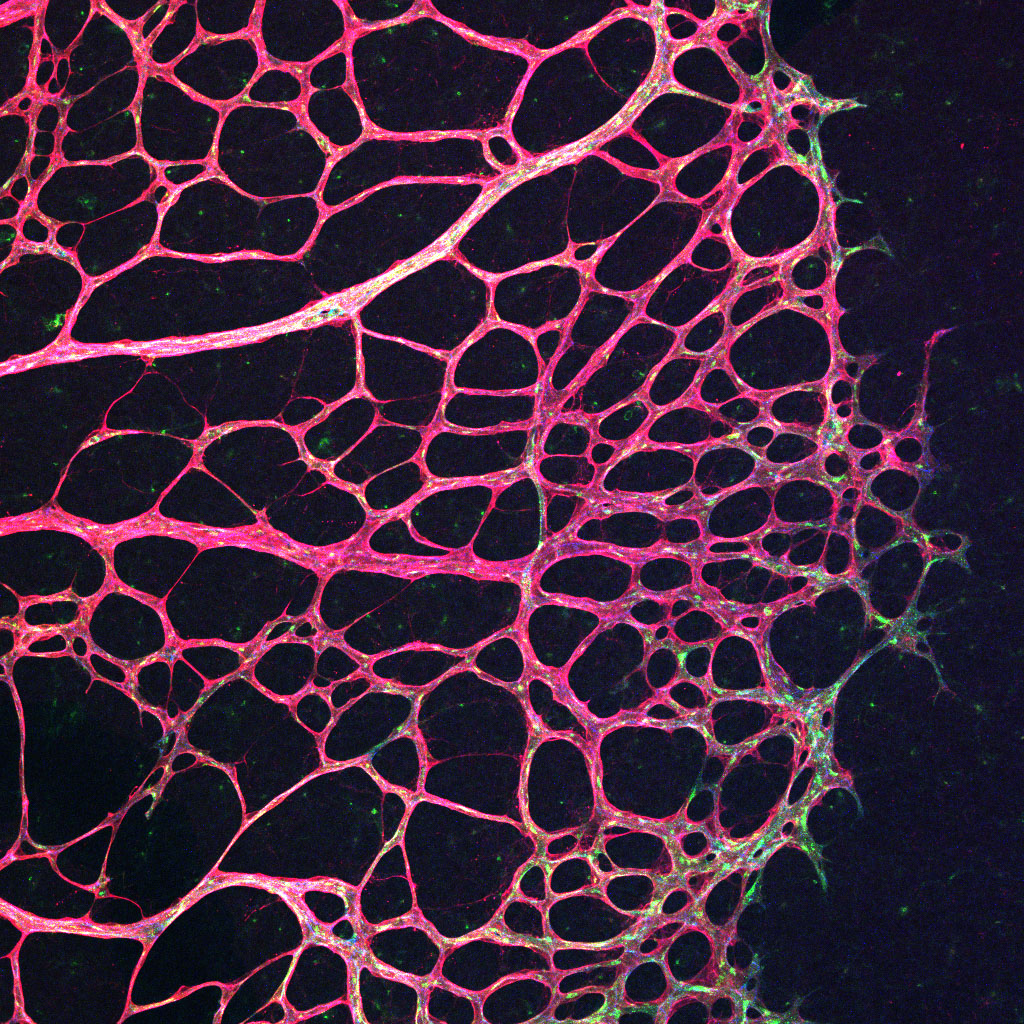
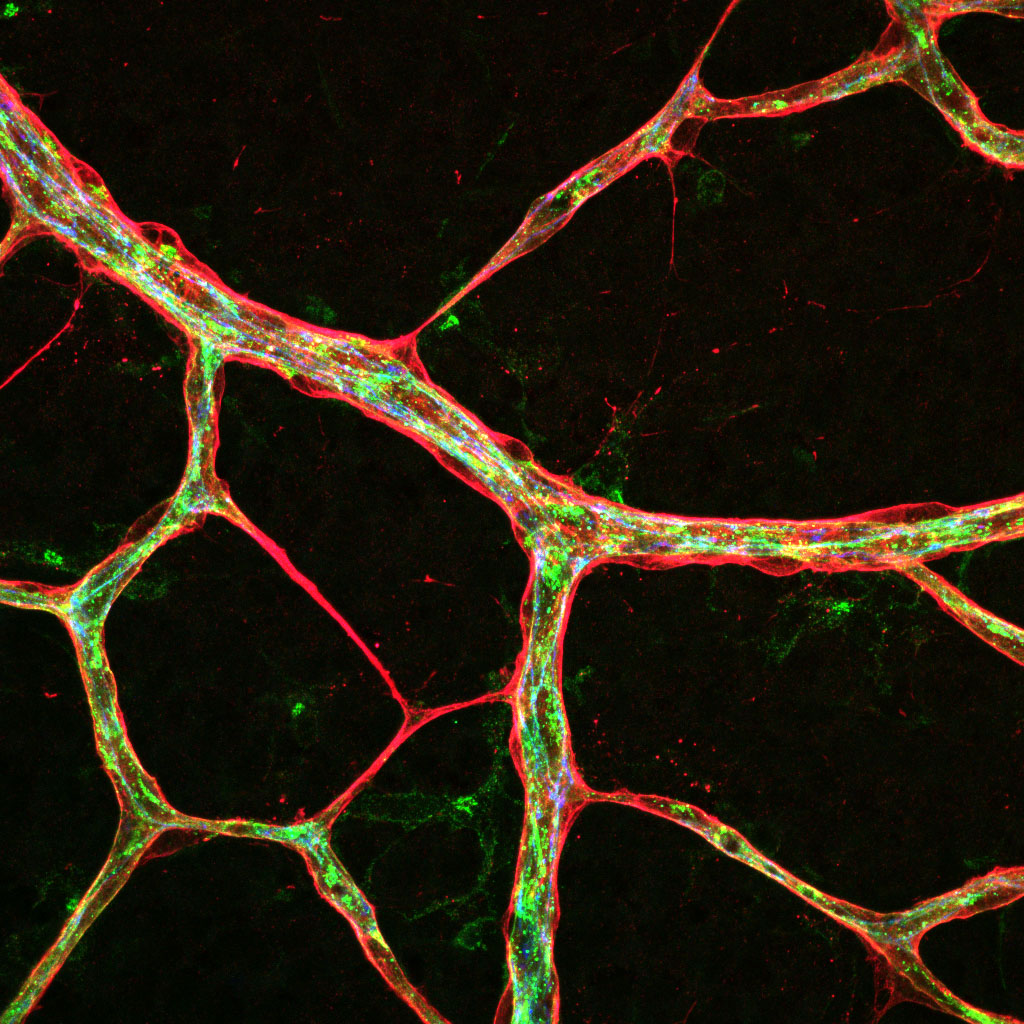
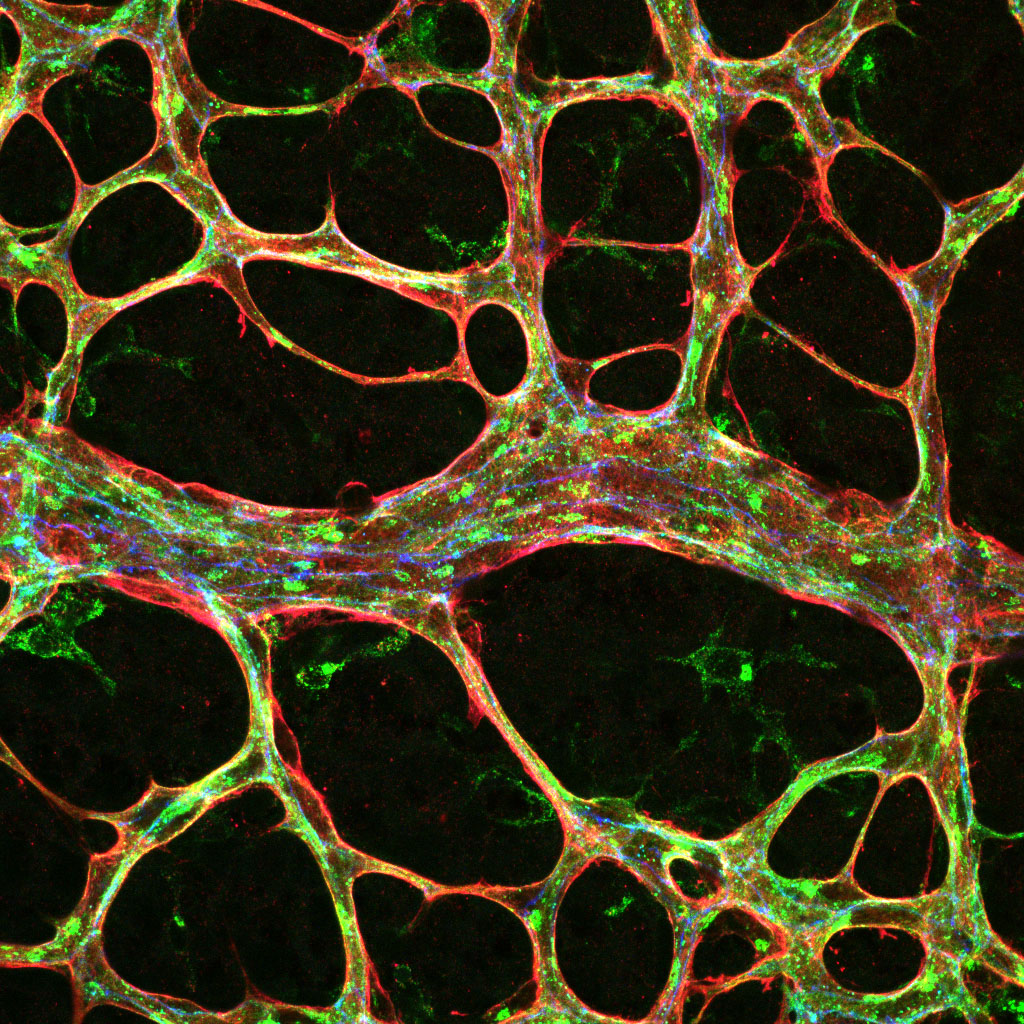
During vertebrate development, vessels originate from one common primitive vascular system, which expands and remodels into a complex, hierarchically organised network of arteries, veins and capillaries. The correct specification, formation and maturation of the 3 distinct vessel subtypes is essential for the vascular network to function, yet the precise cellular processes that underlie vessel divergence remain to be understood.
Objective/mission (the vision)
Arteries carry blood from the heart to the organs of the body and experience high fluid forces and unique stresses, whereas in contrast, veins carry blood back from the body to the heart at a significantly reduced pressure. This exposure to different hemodynamic forces (blood flow) and signalling molecules is reflected by the fact that arteries and veins have inherently different adhesive properties. This project investigates the mechanisms that coordinate the different dynamics of arterial versus venous adhesion that lead to the formation of distinct vessel subtypes.
Research approach (the initiative)
This project utilises a range of novel genetic zebrafish and mouse models, 3D bioengineered vessels, and high-resolution live imaging approaches to identify how adhesion is controlled in different vessel subtypes.
Impacts and applications
Unravelling the molecular determinants controlling how adhesiveness is regulated in order to make a hierarchical, functional network will be crucial pieces of the puzzle for tissue engineering and regenerative medicine approaches in the future to generate vascularised, bioengineered organs. The fundamental knowledge gained from studying regulation of cell-cell adhesion in the vasculature will be applicable in understanding how adhesion regulates the assembly of complex tubular networks in other tissues.
Partners/collaborators
- Dr Anne Lagendijk, IMB/UQ