Bacterial biofilms have been linked to irritable bowel syndrome (IBS), but what exactly are they and how do we get rid of them?
What are biofilms?
A biofilm is a slimy, jelly-like layer of micro-organisms that stick to a surface. Plaque that forms on your teeth and causes tooth decay is an example of a biofilm. They can consist of bacteria, fungi or algae that join together on both living and non-living surfaces in a variety of environments.
A community with benefits
Biofilms host a community of microorganisms, but what do they gain by clubbing together?
Being part of a biofilm provides protection and helps to stick to surfaces – biofilms are much more resistant to hostile environments than free-floating bacteria, enabling microbes to avoid host defences, antibiotics and shear forces.
In fact, biofilms can make bacteria 1000 times more resistant to antibiotics, which is particularly problematic when they grow on medical devices such as replacement hips, to which biofilms can – and do – easily stick.
Biofilms further benefit their individual microbes by taking on some characteristics of a multicellular organism – communicating and sharing genes and nutrients.

Some are ‘good bacteria’
Not all biofilms are problematic for our health, and some ‘good’ bacterial biofilms protect us by competing with ‘bad’ bacteria for space and nutrients in our gut.
Our microbiome in the GI tract
The human gastrointestinal (GI) tract, which extends from the mouth to the anus, is the most densely inhabited environment of the human body, harbouring an abundance of microorganisms with different lifestyles called the gut microbiota. The microorganisms in our gut account for approximately 30 per cent of the human microbiome.
The bacterial density increases along the GI tract, with the highest density in the colon. Many gut microbes live as free-floating cells, but given the high number of microorganisms in the gut, it is perhaps not surprising that others join together to form biofilms.
Linking biofilms with IBS
Associate Professor Markus Muttenthaler is a medicinal chemist at IMB and one of the researchers of a recent clinical study that observed a high prevalence of biofilms in people suffering from irritable bowel syndrome (IBS) and irritable bowel diseases (IBD).
“We found that biofilms were present in 57 per cent of patients with IBS and 34 per cent of patients with ulcerative colitis compared to only 6 per cent of controls, so we think biofilms play an important role in gut disease development – we still do not know if it is a trigger or a consequence, but there is a clear association.”
Biofilms have also been linked to gastric and colorectal cancers in the past.
What are IBS and IBD?
Irritable bowel syndrome (IBS) is a common chronic condition of the gastrointestinal tract. The symptoms include abdominal pain, bloating, and dysregulated bowel movements such as diarrhoea and constipation.
Irritable bowel diseases (IBD) include two chronic inflammatory diseases – ulcerative colitis and Crohn’s disease – both of which are characterised by severe inflammation in the GI tract.
IBS and IBD affect more than 10 per cent of the Western population and represent a substantial socioeconomic problem with limited treatment options.

How do biofilms form in the gut?
Dr Muttenthaler said that in the beginning, you probably would not notice a biofilm forming and spreading.
“The growth of biofilms in our gut is a very slow process, but some of these biofilms can become very big – they can take up large areas of the small intestine and colon.
How do biofilms affect IBS and IBD?
“Once a biofilm has expanded, it becomes a barrier to absorption of bile acids or other molecules in the gut, which can trigger diarrhoea or cause other symptoms. Furthermore, biofilms feed on the mucus layer, which can lead to inflammation,” Dr Muttenthaler said.
“People with these disorders have dysbiosis of the gut microbiome – an unbalanced and less diverse presence of gut bacteria. Sometimes, they can have bacterial overgrowth of a specific species that can form biofilms. We still do not know if these biofilms are the trigger for disease or just a consequence of that microbial imbalance.”
“And as biofilms take up more room, the ‘good’ bacteria can’t penetrate, and it becomes a fight for space and nutrients. And antibiotic treatments further favour this imbalance because biofilms often have high levels of antibiotic resistance.”
A new treatment for IBS
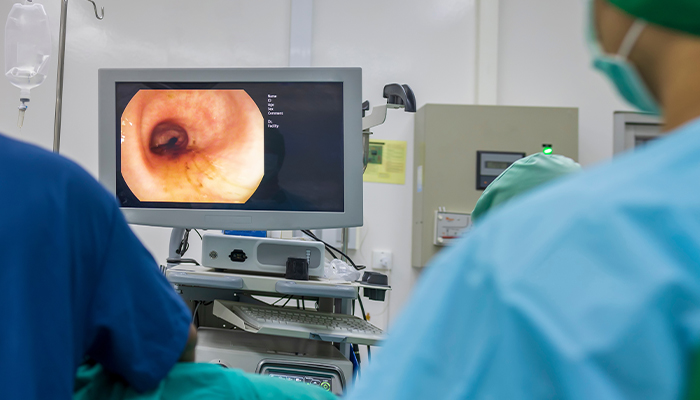
Jet washing with salt water during a colonoscopy can remove some of the larger visible biofilms from the gut and relieve symptoms of IBS and IBD. While early results are promising, this still is an invasive treatment option, and it is unclear how fast these biofilms can come back.
“Biofilms can be flushed out during endoscopy or colonoscopy, using a high-pressure water outlet on the side of the endoscopic camera,” Dr Muttenthaler said.
“The high-pressure water flush can peel biofilms off the gut wall despite their stickiness.
“Patients who have undergone this treatment have had some relief from symptoms for months after.
“It is not a perfect solution because this is an invasive treatment, and there are a lot of other factors that affect these diseases – genetics, diet, anxiety, stress – but the discovery of such bacterial biofilms breaks new ground for therapeutic strategies, and we are currently pursuing more long-term and non-invasive treatment options.
“In the meantime, we want to raise awareness of the presence of such gut biofilms, which are still often misidentified as incomplete bowel preps during colonoscopies. With more gastroenterologists being aware of biofilms and how to remove them, we hope to provide relief to some of the patient population affected by such biofilms.”
Treatments inspired by nature
Researchers at IMB are determined to find treatments that are less invasive and longer-term.
“There are many ways we can try to disassemble biofilms: target their proteins, dissolve their extracellular matrix, interrupt biofilm communication signals and find ways of helping antibiotics to better penetrate such biofilms,” Dr Muttenthaler said.
“We are also looking to nature, to animals that have to naturally defend against biofilms and have developed their own suite of molecules against biofilms, so we can re-create them.”
“We have found antimicrobial peptides on frog skins, which are constantly wet and a prime location for biofilm growth, and inside marsupials’ pouches – a warm, wet environment, again perfect for biofilms to grow.”
“So, we are looking at kangaroos, wallabies and the platypus for clues – they have to fight off parasites and bacterial infection on a constant basis – and we also have some leads from sheep and cow gut.”

Dr Muttenthaler said that once the team has found active peptides, the next challenge is to make them stable for the human gut, with the ultimate goal being to produce an oral drug that can specifically target biofilms without affecting the rest of the gut microbiome.
“We hope to make a drug that can remove biofilms and is also preventative to stop regrowth – this should profoundly improve the quality of life for those with gut disorders.”
The clinical study revealing biofilms in IBS and IBD, as well as a recent review covering this area of research, were published in Gastroenterology.

