The cells in our body move in mysterious ways, adapting themselves to navigate various obstacles in crowded tissues to perform everyday functions.
Navigating a complex 3D world, for example, might require a cell to fit through a tiny pore in the surrounding matrix – a large mesh-like network of proteins that provides structure to tissues –, along tissue tracks, or in between cells– all extremely confined spaces that require squeezing or compression of cells.
IMB's Dr Samantha Stehbens said much of this cellular journey through the crowded tissue environment remains shrouded in mystery.
Defining a new set of principles
But her team’s latest research – published this week in Nature Cell Biology – has defined a new set of principles that will have ongoing impact about the way we think cells experience that compression.
An ARC Future Fellow with joint appointments at IMB and the Australian Institute for Bioengineering and Nanotechnology (AIBN), Dr Stehbens’ research generally revolves around the fundamental mechanisms that regulate cell adhesion and the cytoskeleton.
In this latest study, Dr Stehbens, AIBN's Dr Robert Ju and a multidisciplinary consortium of colleagues sought to understand how cells survive the compression experienced while moving through crowded areas.

Changing shape to fit
Dr Ju said that as cells move throughout our body, they need to protectively adapt their shape to fit through confined spaces – a significant mechanical challenge.
But cells are able to detect mechanical forces from their environment and convert them into biochemical signals – a process termed ‘mechanosensing’.
Cells, like us, move using a skeleton. However, unlike our stiff, stable skeleton, the skeleton inside cells – known as the cytoskeleton –is highly dynamic and constantly remodels to move.
Dr Stebhens, Dr Ju and the team looked at the microtubule cytoskeleton and how it resists compressive loads inside cells as they move.
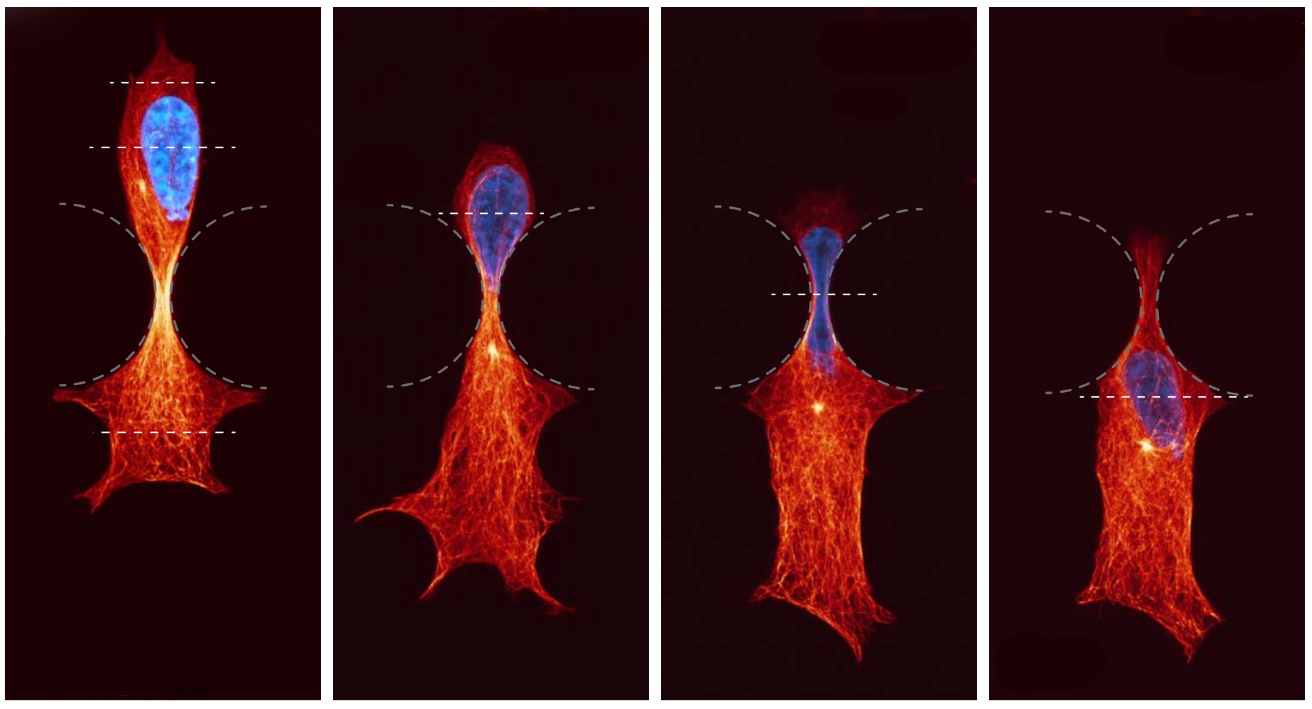
Cellular sensors detect and respond
They described how compression results in the recruitment of microtubule stabilising proteins called CLASPs, to strengthen microtubules – forming a protective armour.
Dr Stehbens said the findings reveal a new role for microtubules as cellular sensors which detect and respond to compressive forces, enabling movement and ensuring cell survival in mechanically demanding environments.
“The molecular mechanisms explored in this project are functional in almost all cell types.
“Hence, the new knowledge generated could have broad impact across model systems and in vivo, beyond cell migration."
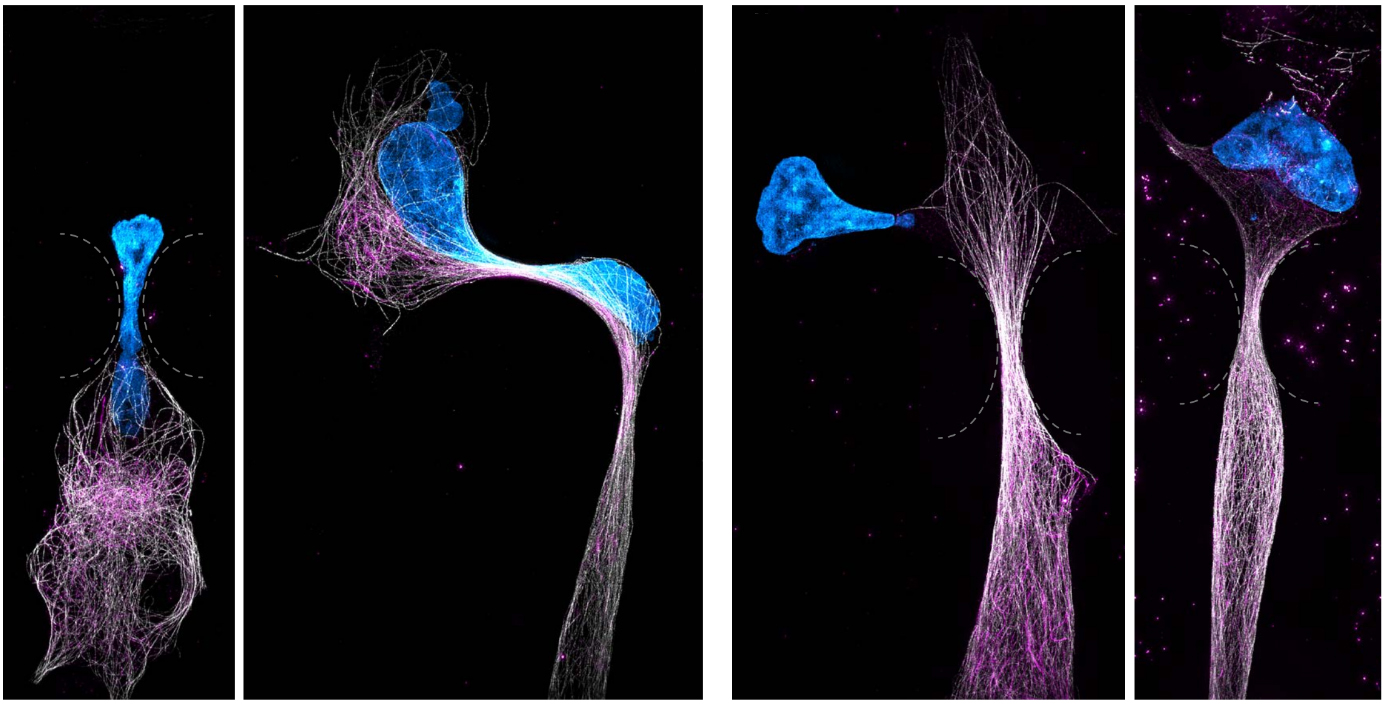
Applying knowledge to disease
"We hope to apply this knowledge to our understanding of disease states. For example in osteoarthritis – where we could strengthen cells responsible for the maintenance of our joints to resist compressive loads – or in the prevention of cancer metastasis – where we could make cancer cells weak and unable to survive the journey to distant organs.
“It will be invaluable for future research in tissue engineering, regenerative medicine and immune function.”
Contributing to this project were researchers from AIBN and IMB, as well as the UQ School of Mathematics and Physics, the UQ Frazer Institute, the University of Texas Southwestern Medical Centre, St. Vincent's Clinical School at the University of Sydney, and the University of Vienna.